Benzoxazole-2-ethyl oxime derivate, preparation method and application thereof
A technology of benzoxazole and ethyl oxime, which is applied in the field of pharmaceuticals, can solve problems such as serious side effects, expensive research and development costs, hidden dangers of kidney and cardiovascular safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
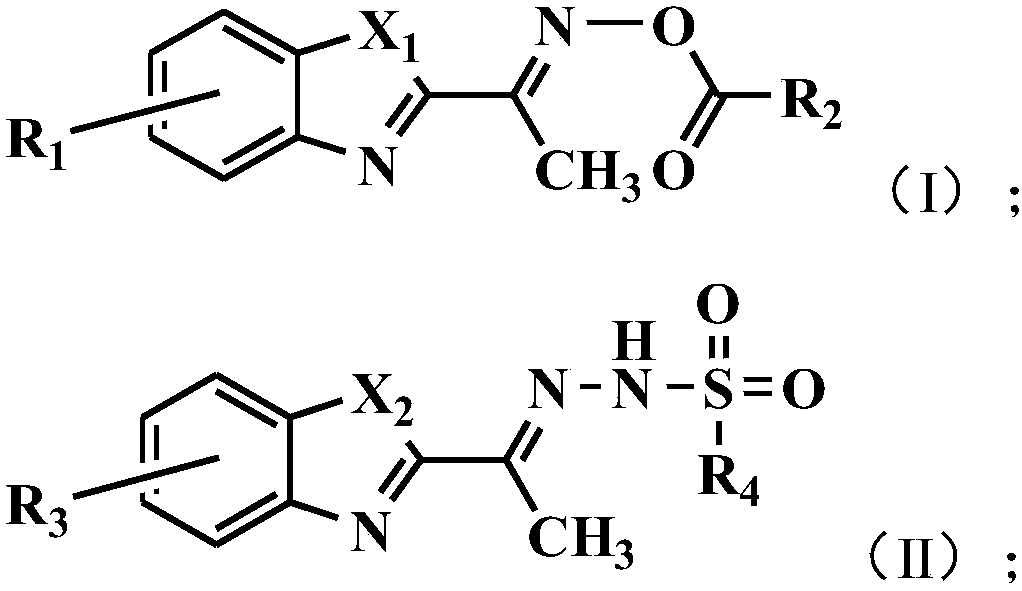
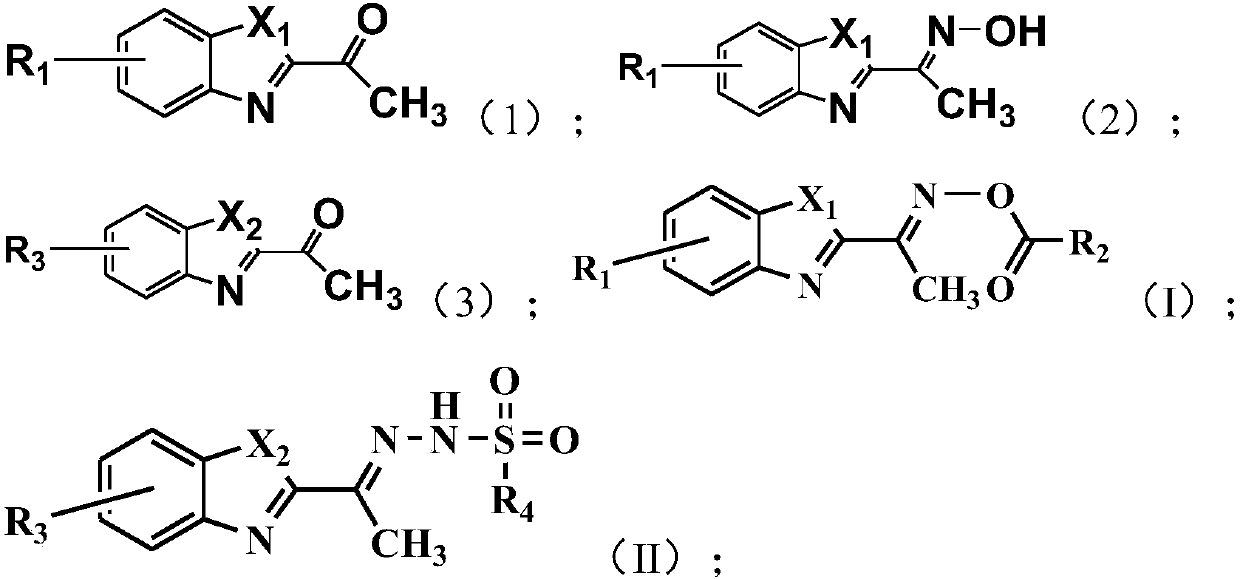
[0041] The present invention also provides a method for preparing the above-mentioned benzoxazole-2-ethyloxime derivatives, comprising:
[0042] Carry out oximation reaction with the compound shown in formula (1) and hydroxylamine hydrochloride, obtain the compound shown in formula (2);
[0043] The compound shown in the formula (2) and R 2 COCl reaction, obtains the compound shown in formula (I);
[0044] Or the compound shown in formula (3) and R 4 SO 2 NHNH 2 Reaction, obtains the compound shown in formula (II);
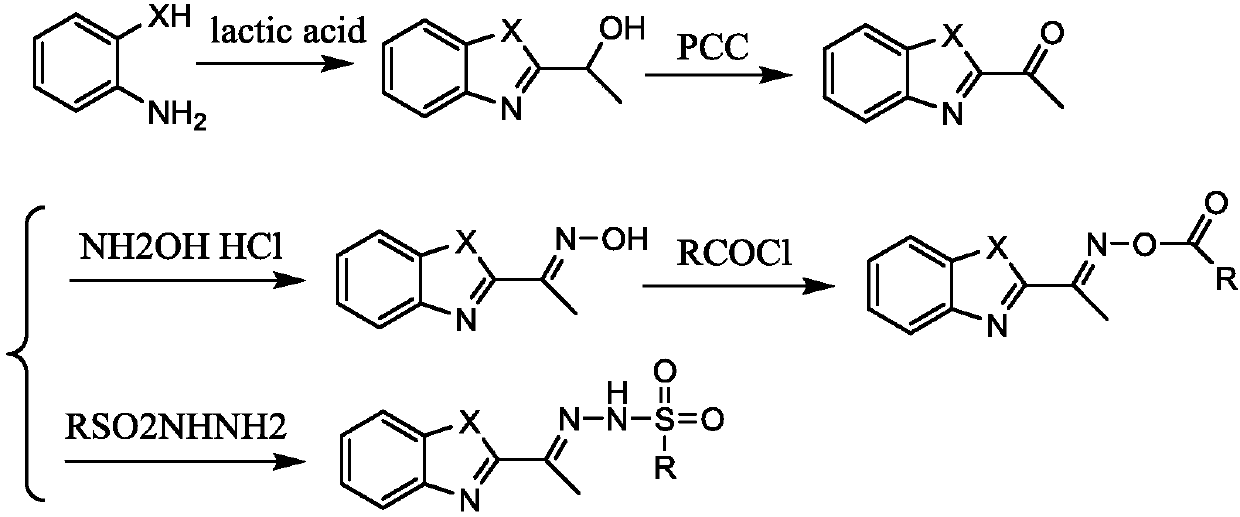
[0045]
[0046] Among them, X 1 with X 2 each independently selected from NH or O;
[0047] R 1 with R 3 each independently selected from H, F, Br or Cl;
[0048] R 2 Selected from C1~C10 alkyl, substituted C1~C10 alkyl, phenyl or substituted phenyl; the substituent of the substituted C1~C10 alkyl is selected from phenyl or halogen; the substituted phenyl The substituents are selected from C1~C10 alkyl, C1~C10 alkoxy, halogen or nitro;
[0049] R ...
Embodiment 1
[0058] Embodiment 1: the synthesis of 1-(benzoxazol-2-ethyl)-1-alcohol
[0059] Add 6g of 2-aminophenol, 5ml (D / L) lactic acid, 20ml of p-toluenesulfonic acid, and 25ml of toluene into a 50ml round-bottom flask, use a water trap, and heat to reflux at 142°C for 8h. After the reaction was finished, wait for the mixture to cool to room temperature, add 100ml of water, extract with ethyl acetate, extract 3 times with 20ml each time, dry with anhydrous magnesium sulfate, and spin evaporate. The crude product was passed through the column with petroleum ether: ethyl acetate (4:1 ~ 2:1) gradient to obtain a viscous tan product.
[0060] Utilize nuclear magnetic resonance to analyze the product obtained in embodiment 1, obtain: 1 HNMR (600MHz, DMSO-d 6 )δ7.72(t, J=8.6Hz, 2H), 7.37(qd, J=10.3, 6.9, 6.0Hz, 2H), 5.93(d, J=5.0Hz, 1H), 4.96(p, J=5.9 Hz, 1H), 1.54 (t, J=4.2Hz, 3H).
[0061] 13 C NMR (151MHz, DMSO-d 6 ) δ 168.78, 150.50, 140.91, 125.56, 124.81, 120.18, 111.26, 62.93, ...
Embodiment 2
[0062] Embodiment 2: Synthesis of 1-(benzoxazol-2-ethyl)-1-ketone
[0063]Dissolve 1g of compound 1-(benzoxazol-2-ethyl)-1-ol in 50ml of dichloromethane, mix 1.3g of pyridinium chlorochromate with 6.5g of silica gel powder, add the above solution, and stir overnight at room temperature , filtered after the reaction, and rinsed the residue with acetone, collected the filtrate and rotary evaporated. The crude product was separated by column with petroleum ether: dichloromethane (1:1) to obtain a white to light yellow product.
[0064] Utilize nuclear magnetic resonance to analyze the product obtained in embodiment 2, obtain: 1 HNMR (600MHz, Chloroform-d) δ7.90 (dt, J=8.0, 1.0Hz, 1H), 7.68–7.64 (m, 1H), 7.54 (ddd, J=8.3, 7.2, 1.2Hz, 1H), 7.46 (ddd, J = 8.3, 7.3, 1.1 Hz, 1H), 2.82 (s, 3H).
[0065] 13 C NMR (151MHz, DMSO-d 6 )δ 152.6, 150.0, 140.1, 110.6, 119.1, 123.8, 124.8, 185.0, 26.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com