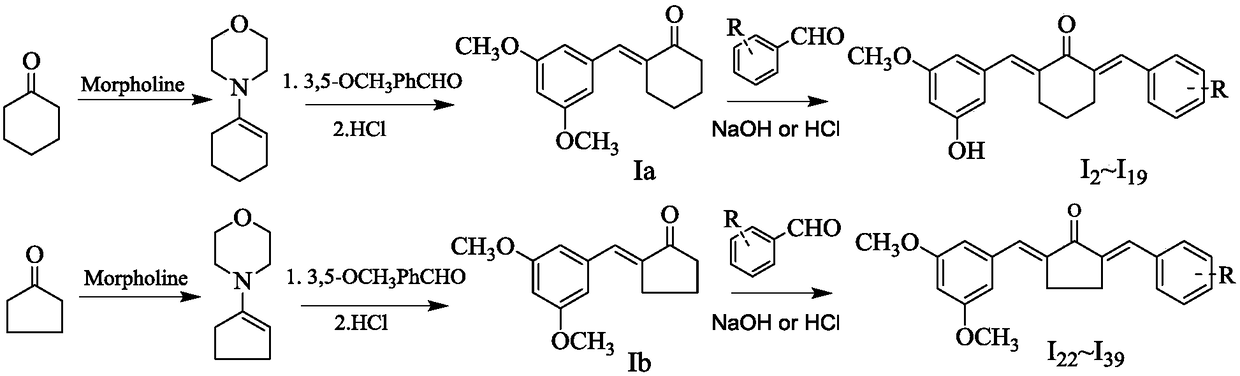
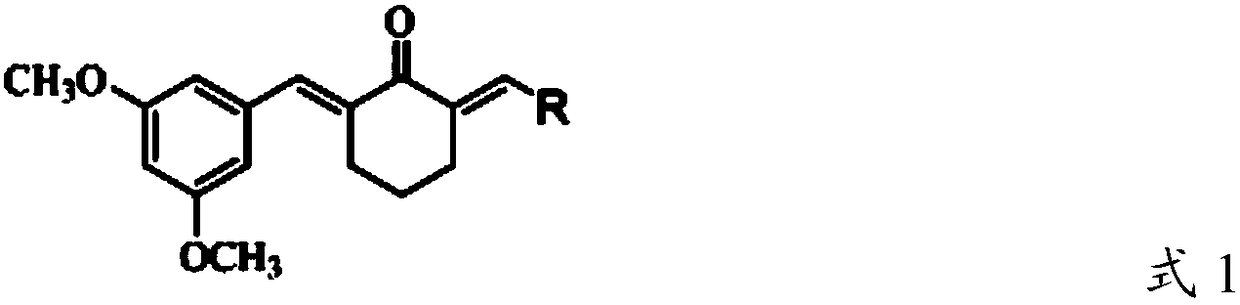
New applications of (2e, 6E)-2-(3,5-dimethoxyphenylmethylene)-6-(4-chlorophenylmethylene)cyclohexanone and its derivatives
A 4-N, 2-clph technology, applied in the directions of medical preparations, drug combinations, organic active ingredients, etc. containing active ingredients, can solve the problems of acute attack of gout without treatment effect, serious side effects, and many side effects, etc. Reduce ankle swelling and serum uric acid level, good dual inhibitory activity, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, E-2-(3,5-dioxybenzyl)-cyclohexanone (I a ) and E-2-(3,5-dioxybenzyl)-cyclopentanone (I b )Synthesis:
[0027] Add cyclohexanone or cyclopentanone (0.11mol) and morpholine (10.4g, 0.12mol) to 20mL of benzene, install a water separator, reflux and azeotrope until anhydrous is formed, evaporate benzene and morpholine under reduced pressure phyllolines to give enamines. Add enamine (6.54g, 0.043mol) and 3,5-dimethoxybenzaldehyde (5.40g, 0.033mol) to 20mL of benzene, install a water trap, and reflux to azeotrope until no water is formed. A total of 8 hours, cooled to room temperature, slowly added 6mg / L hydrochloric acid under stirring, stirred at room temperature for 2 hours, separated the benzene layer, extracted the water layer with the benzene layer, combined the benzene layer, dried over anhydrous sodium sulfate, concentrated, petroleum ether and ethanol weight Crystallized yellow needle-like crystals (I a or I b ).
[0028] Will I a or I b(1.22mmo...
Embodiment 2
[0031] Example 2, (2E,6E)-2-(3,5-dimethoxyphenylmethylene)-6-(3-chlorophenylmethylene)cyclohexanone (I 2 )Synthesis
[0032] See Example 1 for the synthesis method.
[0033] Mp: 107.5~108.4℃. 1 H-NMR (400MHz, CDCl 3 ), δ(ppm): 7.72(s,1H,=CH),7.70(s,1H,=CH),7.43(s,1H,ArH),7.30~7.36(m,3H,ArH),6.61(d ,2H,J=2.1Hz,ArH),6.47(t,1H,J=2.1Hz,ArH),3.82(s,6H,-OCH 3 ),2.88~2.95(m,4H,-CH 2 ), 1.81 (quint, 2H, J=6.5Hz, -CH 2 ).
[0034] 13 C-NMR (400MHz, CDCl 3 ), Δ (PPM): 190.257,160.841,137.985,137.876,137.577,137.541,136.631,134,134.564,172,128.795, 101.1062,8.70.70.70.70.7062,8,8,70.70.70.70.70.7062,8,8,70.70.70.70.70.7062,8,8,70.70.70.70.70.7062,8.70.7062,8,70.70.7062,8,70.
[0035] HR-MS: Calcd. For C 22 h 21 ClO 3 [M+H] + :369.1252,Found:369.1257.
Embodiment 3
[0036] Example 3, (2E,6E)-2-(3,5-dimethoxyphenylmethylene)-6-(2-chlorophenylmethylene)cyclohexanone (I 3 )Synthesis
[0037] See Example 1 for the synthesis method.
[0038] Mp: 104.0~104.7℃. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):7.88(s,1H,=CH),7.74(s,1H,=CH),7.43~7.45(m,1H,ArH),7.32~7.34(m,1H,ArH),7.27 ~7.30(m,2H,ArH),6.61(d,2H,J=2.0Hz,ArH),6.47(t,1H,J=2.1Hz,ArH),3.82(s,6H,-OCH 3 ),2.94(t,2H,J=5.6Hz,-CH 2 ),2.76(t,2H,J=5.5Hz,-CH 2 ), 1.77 (quint, 2H, J=6.2Hz, -CH 2 ).
[0039] 13 C-NMR (400MHz, CDCl 3 ),δ(ppm):190.280,160.837,138.169,137.948,137.752,136.739,135.260,134.694,133.913,130.818,130.009,129.827,126.555,108.627,101.090,55.706,28.983,28.483,23.363.
[0040] HR-MS: Calcd. For C 22 h 21 ClO 3 [M+H] + :369.1252,Found:369.1251.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com