Polymorphic substance of 5-(2-fluorophenyl)-N-methyl-(3-pyridylsulfonyl)-1H-pyrrole-3-methyl ammonium acetate
A technology of pyridylsulfonyl and carbamate, which can be applied in drug combinations, medical preparations containing active ingredients, organic chemistry, etc., and can solve the effects of poor water solubility of compounds, limiting acid suppression and treating gastric acid-related diseases and other issues to achieve good compression and good effect on lighting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Crystal Form A
[0042] In a 100 mL one-necked bottle, add 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methanamine (5.0 g, 14.49 mmol) and Ethyl acetate (25 mL) was stirred and dissolved at room temperature (25°C), and glacial acetic acid (0.5 mL, 17..39 mmol) was added dropwise to the reaction system, stirred at this temperature for 60 min, solids were precipitated, and the temperature was lowered to 0-5°C, continue stirring for 1 h to filter out the solid, and wash with ethyl acetate (10 mL*2 times), and dry the obtained solid in a vacuum oven (35°C) for 4 hours to obtain 4.3 g of off-white solid, The yield was 74%.
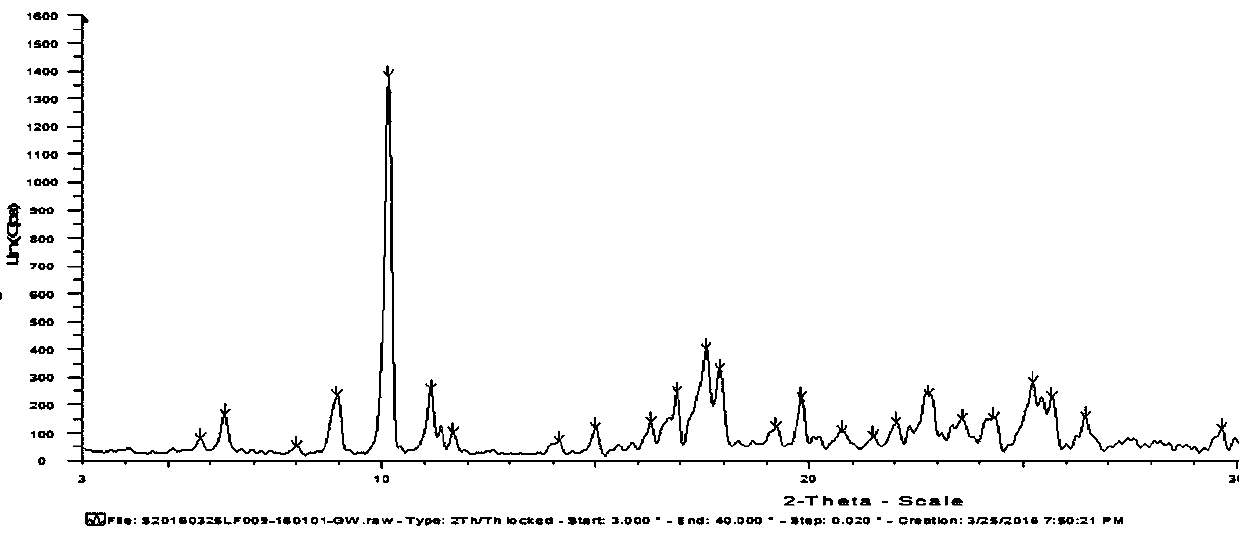
[0043] The A crystal form of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-carbamate was subjected to powder X-ray diffraction, scanning range In the 2θ interval from 3° to 40°, the crystal structure of the compound was obtained, test conditions: 40kv 40mA; slit: 1.0 / 1.0 / Ni / 0.1; step size: 0.02°; target typ...
Embodiment 2
[0045] Example 2 Crystal Form B
[0046] In a 100 mL one-necked bottle, add 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methanamine (5.0 g, 14.49 mmol) and Ethyl acetate (25 mL) was stirred and dissolved at room temperature (25 °C), and glacial acetic acid (0.5 mL, 17.39 mmol) was added dropwise to the reaction system, stirred at this temperature for 30 min, solids were precipitated, stirred and heated to 50 ±5°C, keep warm for 1h, then lower the temperature to 2°C, and stir for 30 minutes, filter the solid, wash with ethyl acetate (20 mL*2 times), and dry the obtained solid in a vacuum oven (35°C ) for 4 hours to obtain 3.8 g of off-white solid with a yield of 65.5%.
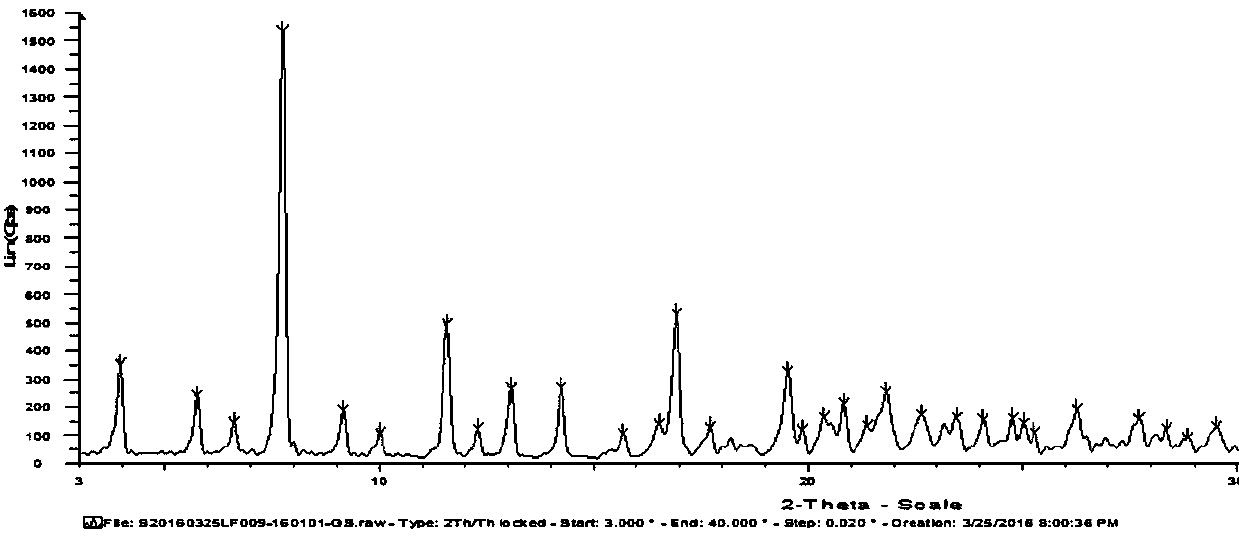
[0047] The B crystal form of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-carbamate was subjected to powder X-ray diffraction, scanning range In the 2θ interval from 3° to 40°, the crystal structure of the compound was obtained, test conditions: 40kv 40mA; slit: 1.0 / 1.0 / Ni...
Embodiment 3
[0049] Example 3 Crystal Form C
[0050]In a 100 mL one-necked bottle, add 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methanamine (5.0 g, 14.49 mmol) and Tetrahydrofuran (50 mL) was stirred and dissolved at room temperature (25°C), and glacial acetic acid (0.5 mL, 17.39 mmol) was added dropwise to the reaction system, stirred and heated to 60°C, and stirred at this temperature for 30 minutes, then cooled to 40°C, keep stirring for 5h, then cool down to 0±5°C, and stir at this temperature for 30min, filter with suction, wash with THF (30 mL*2 times), and dry the obtained solid in a vacuum oven (35°C) 4 hours, 2.6 g of off-white solid was obtained, and the yield was 44.8%.
[0051] The C crystal form of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-carbamate was subjected to powder X-ray diffraction, scanning range In the 2θ interval from 3° to 40°, the crystal structure of the compound was obtained, test conditions: 40kv 40mA; slit: 1.0 / 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com