Method for regenerating fluorination catalyst
A technology of fluorination catalyst and hydrogen fluoride, which is applied in the direction of catalyst regeneration/reactivation, physical/chemical process catalysts, chemical instruments and methods, etc. It can solve the problems of rapid decline in activity of fluorination catalysts, reduced activity, and easy loss of active metal chromium, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
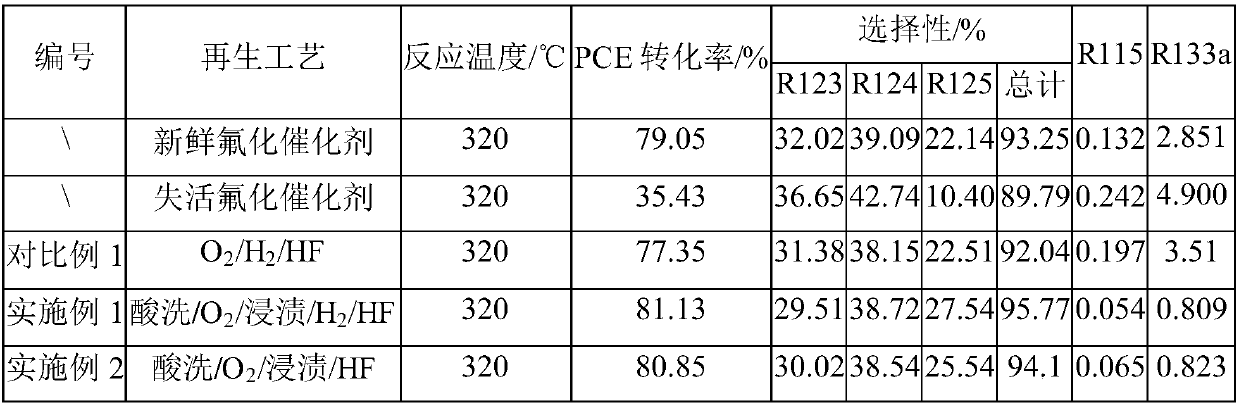
[0073] Preparation of fresh fluorination catalyst
[0074] Weigh 500g CrCl 3 ·6H 2 O, 4.56g MgCl 2 , 4.42g InCl 3 , 10.13g CoCl 2 Dissolve in 11.45L deionized water, mix the mixed salt solution and ammonia water until the pH of the solution is 9, filter and wash, put the sample into an oven and dry it at 110°C for 6 hours, then transfer it to a roasting furnace, in N 2 Calcined at 350°C for 4 hours in the atmosphere, crushed and sieved the calcined sample, added 2% graphite (mass fraction) additive to mix evenly, and pressed into tablets to obtain a catalytic precursor.
[0075] Then, the above-mentioned catalyst precursor was loaded into a reactor, and a mixed gas of nitrogen and HF was introduced to fluorinate at 350° C. to prepare a fresh fluorination catalyst.
[0076] Preparation of deactivated fluorination catalyst
[0077] After the fresh fluorination catalyst was reacted for 1600 hours, the catalyst was heated to 300° C., and nitrogen gas at a temperature of 300°...
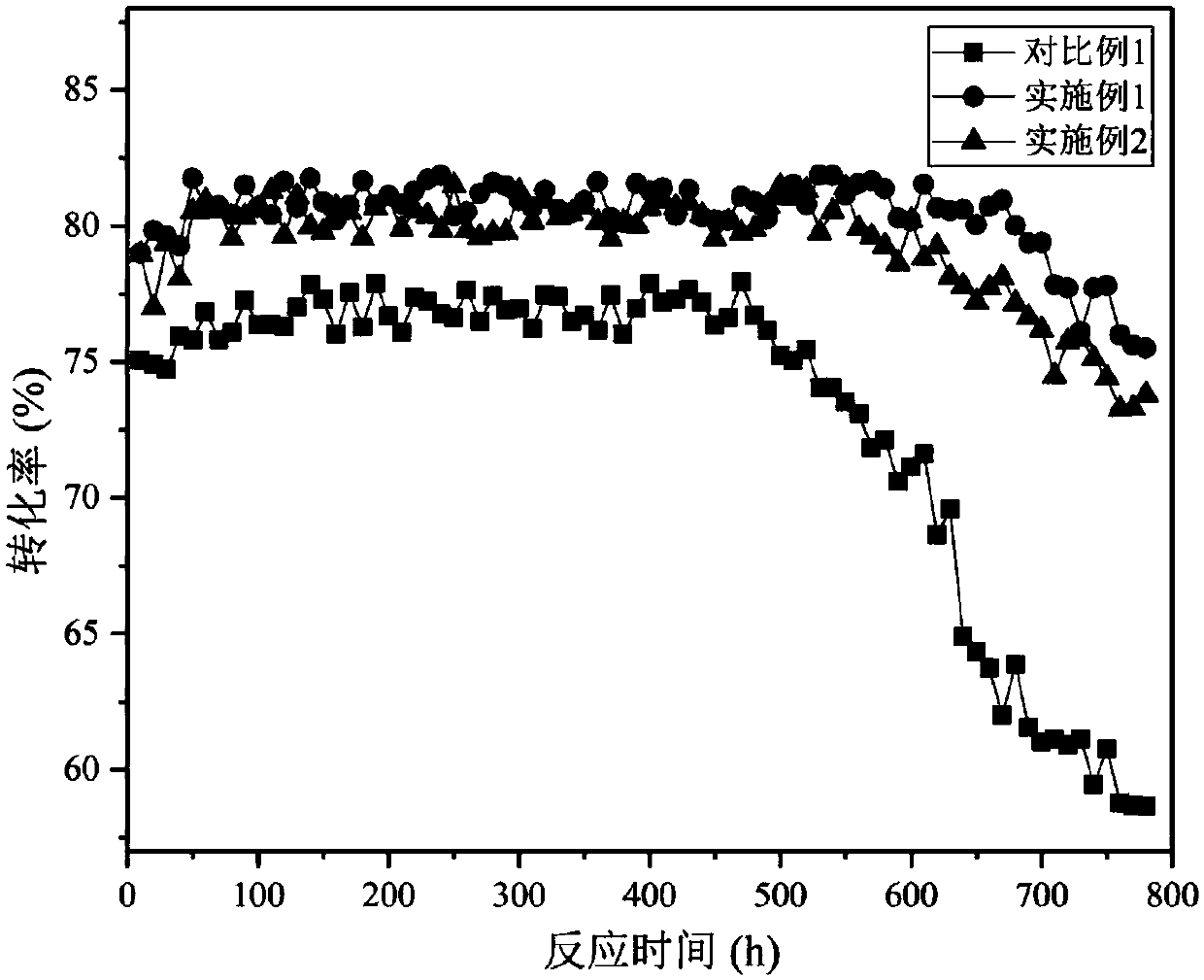
Embodiment 1
[0083] Soak the deactivated fluorination catalyst in a mixed solution of 10% hydrofluoric acid and 10% nitric acid for 2 hours, take it out, and dry it in an oven at 120°C for 10 hours;
[0084] The above-mentioned acid-washed fluorination catalyst was packed into a self-made fixed bed, and nitrogen gas with a temperature of 300° C. was introduced to purge the fluorination catalyst for 25 hours; nitrogen and oxygen (mass ratio 15° C. : 1) the mixed gas is calcined, the aeration time is 20h, thereafter the oxygen ratio is gradually increased until it is pure oxygen, and the temperature fluctuation of the fluorination catalyst is controlled to be less than 10°C, and the pure oxygen aeration time is 20h;
[0085] The above-mentioned calcined fluorination catalyst was placed in MgCl 2 and CrCl 3 The mixed solution (among them, MgCl 2 The mass percentage is 10%, CrCl 3 The mass percentage is 60%), soaked in 4h, take out, put in oven and dry at 120 ℃ for 10h;
[0086]Put the abo...
Embodiment 2
[0089] Soak the deactivated fluorination catalyst in a mixed solution of 10% hydrofluoric acid and 10% nitric acid for 2 hours, take it out, and dry it in an oven at 120°C for 10 hours;
[0090] The above-mentioned acid-washed fluorination catalyst was packed into a self-made fixed bed, and nitrogen gas with a temperature of 300° C. was introduced to purify the fluorination catalyst for 30 h; under normal pressure, the temperature was 300° C. nitrogen and oxygen (mass ratio 15: 1) The mixed gas is calcined, the aeration time is 20h, and then the proportion of oxygen is gradually increased until it is pure oxygen, and the temperature fluctuation of the fluorination catalyst is controlled to be less than 10°C, and the pure oxygen aeration time is 20h;
[0091] The above-mentioned calcined fluorination catalyst was placed in MgCl 2 and CrCl 3 The mixed solution (among them, MgCl 2 The mass percentage is 10%, CrCl 3 The mass percentage is 60%), soaked in 4h, take out, put in ov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com