Synthesis method of trajenta intermediate
A synthetic method and intermediate technology, which is applied in the field of synthesis of linagliptin intermediates, can solve the problems of long routes and low yields, and achieve the effects of high yields, fewer steps, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
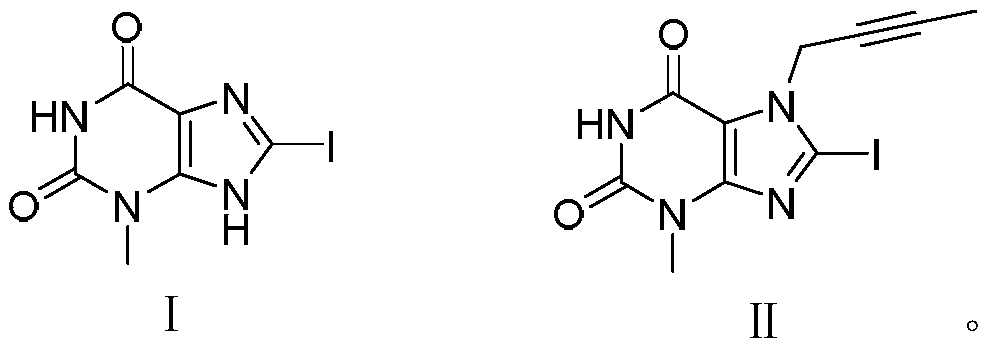
[0024] Embodiment 1, the preparation of formula I compound
[0025]
[0026] In a 50mL single-necked bottle, add successively, 1-methyluracil (1.26g, 10mmol), urea (1.20g, 20mmol), potassium iodide (1.83g, 11mmol), TBHP (70%, 2.83g, 22mmol), Sulfuric acid (concentration 98wt%, 49mg, 0.5mmol), 20mL of dioxane, then raise the temperature to 75°C and stir for 18h. Afterwards, 80 mL of ethyl acetate was added to dilute the mother liquor, washed three times with saturated aqueous sodium sulfite solution, once with saturated aqueous sodium chloride solution, and finally washed once with water, and the ethyl acetate was rotary evaporated under reduced pressure to obtain the crude product. The crude product was recrystallized from dichloromethane and cyclohexane to obtain 2.39 g of compound I with a yield of 82% and a purity of 99%.
Embodiment 2
[0027] Embodiment 2, the preparation of formula II compound
[0028]
[0029] In a 100mL single-necked bottle, add formula I compound (2.92g, 10mmol), N, N-diisopropylethylamine (1.94g, 15mmol), 1-bromo-2-butyne (1.98g, 15mmol) , 30mL of acetone, heated to 50°C, and refluxed for 5h. The reaction was cooled, cooled to room temperature, filtered with suction, and the filter cake was washed with methanol to obtain the crude product as a light yellow solid. The crude product was recrystallized with dichloromethane and cyclohexane to obtain 3.23 g of compound II with a yield of 94% and a purity of 99%. .
[0030] EI-SI m / z: 345[M+H]+
[0031] 1H NMR (500MHz, DMSO-d6) δ11.26(s, 1H), 5.00(d, 2H, J=2.5Hz), 3.31(s, 3H), 1.80(t, 3H, J=2.5Hz).
Embodiment 3
[0032] Embodiment 3, the preparation of formula I compound
[0033] Same as Example 1, the only difference is:
[0034] The iodine source is TBAI; the molar ratio of 1-methyluracil to TBAI is 1.0:1.05. The oxidant is iodine; the molar ratio of 1-methyluracil to iodine is 1.0:2.05. The acid catalyst is hydrochloric acid (concentration 37wt%); the molar ratio of 1-methyluracil to hydrochloric acid is 1.0:0.01. The molar ratio of 1-methyluracil to urea is 1.0:1.05. The reaction solvent is DMF; the reaction temperature is 90°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com