TNFr and stability and anti-aggregation performance enhanced Fc fragment fusion protein as well as preparation method and application thereof
A fusion protein and anti-aggregation technology, applied in the field of Fc fragment fusion protein and its preparation, can solve the problems of poor stability and weak anti-aggregation ability, and achieve the effects of good stability, good anti-aggregation ability and reducing the risk of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Amplification of TNFr-Fc and TNFr-S23 fragment genes
[0024] A human blood sample was taken, and the total cell RNA was extracted with Trizol Reagent (Invitrogen), and the obtained total cell RNA was dissolved in 50 μL of RNase-free water. Then use the M-MLV Reverse Transcriptase Kit (Promega Company), using random primers, and reverse transcription to obtain cDNA.
[0025] According to the Fc gene sequence of IgG (GenBank: KJ905798.1), analyze its amino acid sequence and design the forward and reverse primers of the Fc gene to amplify the target fragment:
[0026] Forward primer
[0027] 5-GAG CCC AAA TCT AGC GAC AAAACT CAC AC-3(1)
[0028] 5-ACGC GGCCCAGCCGGCC TTGCCCGCCCAGGTGGCATTTAC-3(2) (the horizontal line is marked as Sfi I restriction site);
[0029] Reverse primer
[0030] 5-GCCCTC CTCGAG TCATTTACCCGGAGACAGGGAG-3(1) (the horizontal line is the Xho I restriction site)
[0031] 5-GTCGCCAGTGCTCCCTTCAGCTGGG-3(2)
[0032] Use forward primers (1) / (2) and reverse pri...
Embodiment 2
[0045] Example 2: Expression and purification of TNFr-Fc and TNFr-S23
[0046] PEI (2mg / mL PEI) (Polyethylenimine "Max", (Mw 40,000)-High Potency Linear PEI, Polysciences, Inc., Catalog No.: 24765-2, 2g) was used for each 40ug of TNFr-Fc and TNFr-S23 plasmids. 293F cells were transfected and cultured with 40ml 293F expression medium (invitrogen) at 37°C and 150rpm shaker for 6 days.
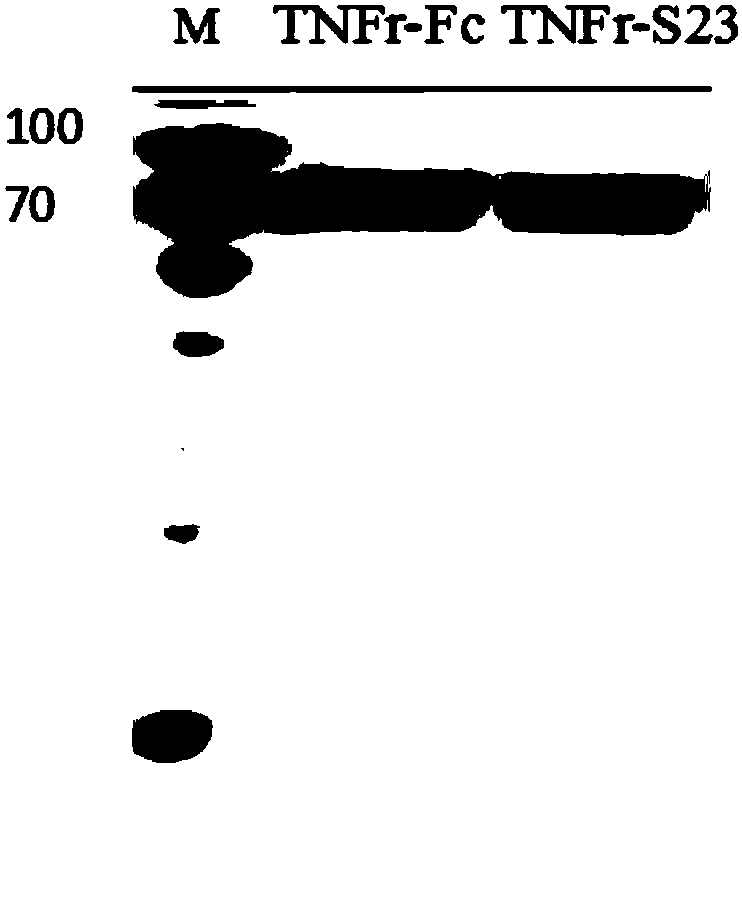
[0047] Subsequently, the supernatant of 293F cells was collected by centrifugation (6000g, 4°C, 15min) (the supernatant uses mouse anti-human IgG Fc as the primary antibody and HRP-labeled goat anti-mouse IgG as the secondary antibody for Western blot detection. The results are as follows: figure 1 As shown, lane 1: Marker; lane 2: TNFr-Fc cell supernatant; lane 3: TNFr-S23 cell supernatant). After the collected supernatant is filtered, TNFr-Fc, TNFr-S23 protein. Subsequently, the target protein was concentrated by ultrafiltration with an ultrafiltration centrifuge tube (Merck Millipore) with a molec...
Embodiment 3
[0048] Example 3: Molecular conformation and existence of TNFr-Fc and TNFr-S23 (monomer, dimer, etc.)
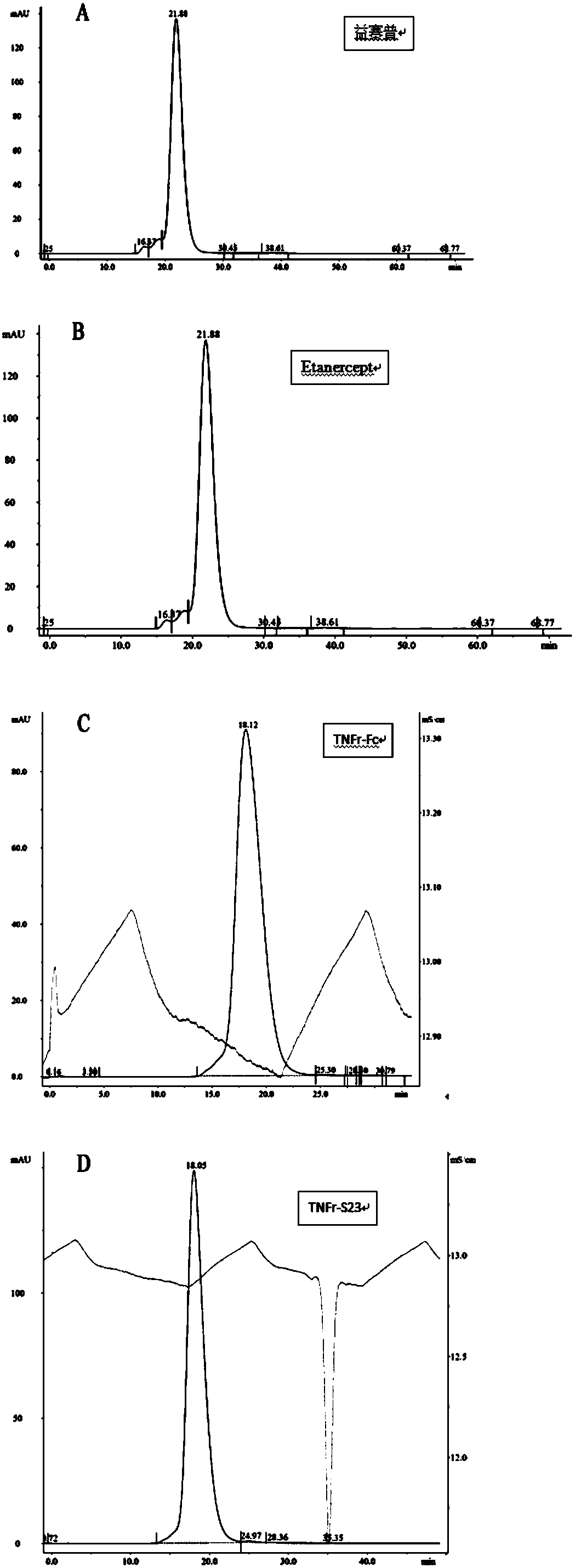
[0049] AKTA analysis of TNFr-Fc and TNFr-S23 existence form: Concentrate the purified TNFr-Fc and TNFr-S23 protein to 1mg / ml, PBS (pH7.4) as the elution buffer, and pass Column Superdex 200 Increase 10 / 300GL , Where the flow rate is 0.5ml / min, and its existence form is detected. At the same time, 1mg / ml Etanercept and Yisaipu protein were also passed through Column Superdex 200 Increase 10 / 300GL molecular sieve to compare the existing forms of the protein.
[0050] The result is image 3 As shown, Yisaipu (A), Etanercept (B), TNFr-Fc (C), TNFr-S23 (D), compared with the standard curve can be found, TNFr-Fc (C), TNFr-S23 (D) protein molecular weight About 140kDa, the results show that they exist in the form of dimers. Compared with the molecular sieve diagrams of Etanercept and Yisaipu protein, the peak of TNFr-S23 is more single, indicating that the protein is more stable and si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com