Method for preparing phenylethanolamine compound intermediate
A technology of phenylethanolamine and compound, applied in the field of chemical synthesis, can solve problems such as many steps, harsh reaction conditions, increasing target product steps and time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of 4-methoxymandelic acid
[0071]
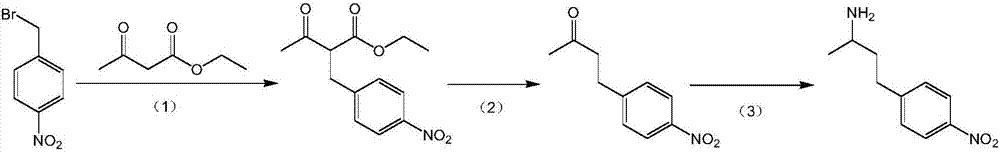
[0072] Add 4-methoxybenzaldehyde (109g, 800mmol), chloroform (140mL) and benzyltriethylammonium chloride (9.12g, 40mmol) into a 1L three-necked flask, and heat the resulting solution to 58°C for 10 minutes Until all the solids were dissolved, then 50% aqueous sodium hydroxide solution (200 mL) was added dropwise into the reaction flask, and the reaction was continued at 58° C. for 30 minutes after the addition was completed. The reactant was cooled to about 5°C, acidified with 50% sulfuric acid, and the resulting aqueous solution was extracted with ether (3×1 L). The organic phase was dried with anhydrous magnesium sulfate, and the solvent was evaporated to obtain 164.1 g of milky white solid (yield: 90%). 1 The H NMR spectrum shows that it is completely consistent with the standard spectrum reported in the literature. Example 2: Preparation of 4-(4-nitrophenyl)-butan-2-amine and 4-(2-nitrophenyl)-b...
Embodiment 2
[0072] Add 4-methoxybenzaldehyde (109g, 800mmol), chloroform (140mL) and benzyltriethylammonium chloride (9.12g, 40mmol) into a 1L three-necked flask, and heat the resulting solution to 58°C for 10 minutes Until all the solids were dissolved, then 50% aqueous sodium hydroxide solution (200 mL) was added dropwise into the reaction flask, and the reaction was continued at 58° C. for 30 minutes after the addition was completed. The reactant was cooled to about 5°C, acidified with 50% sulfuric acid, and the resulting aqueous solution was extracted with ether (3×1 L). The organic phase was dried with anhydrous magnesium sulfate, and the solvent was evaporated to obtain 164.1 g of milky white solid (yield: 90%). 1 The H NMR spectrum shows that it is completely consistent with the standard spectrum reported in the literature. Example 2: Preparation of 4-(4-nitrophenyl)-butan-2-amine and 4-(2-nitrophenyl)-butan-2-amine
[0073]
[0074] Add fuming nitric acid (500mL) in a 1L thre...
Embodiment 3
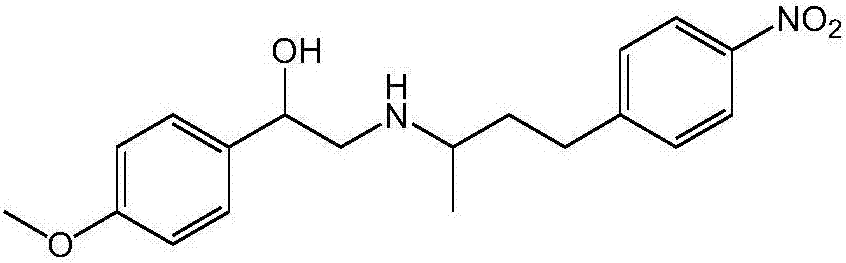
[0076] Embodiment 3: the preparation of PEA intermediate
[0077]
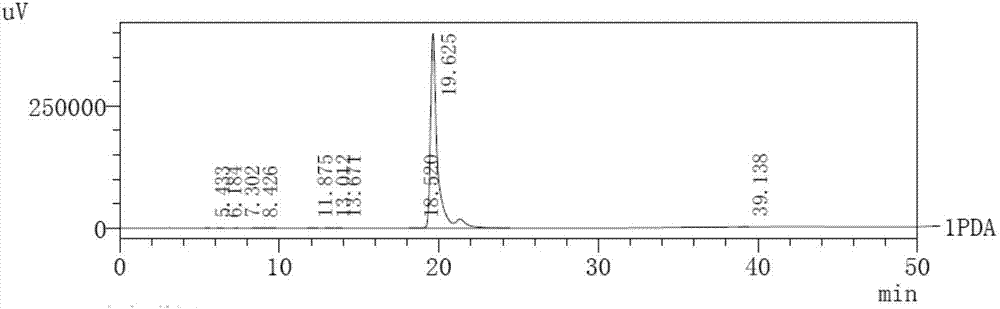
[0078] The mixture (34.2g, 176mmol) of 4-(4-nitrophenyl)-butan-2-amine and 4-(2-nitrophenyl)-butan-2-amine prepared in Example 2, Example 1 The prepared 4-methoxymandelic acid (32g, 176mmol), EDC·HCl (52g, 270mmol), HOBt (36.5g, 270mmol) were dissolved in dichloromethane (300mL), stirred at room temperature for 48 hours until TLC showed The reaction is complete. The reacted mixture was washed with water (2×300 mL), part of the product was crystallized and filtered to obtain 13.3 g of the first batch of product. The filtrate was dried with anhydrous magnesium sulfate, and the solvent was spin-dried, and the obtained oil was washed with acetonitrile (50 mL) to obtain a second batch of product 29.9 g. The two batches of products were combined and recrystallized three times with DMF to remove a small amount of ortho-nitro impurity to obtain 27.6g (yield: 44%) of pure product without ortho-nitro impurity.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com