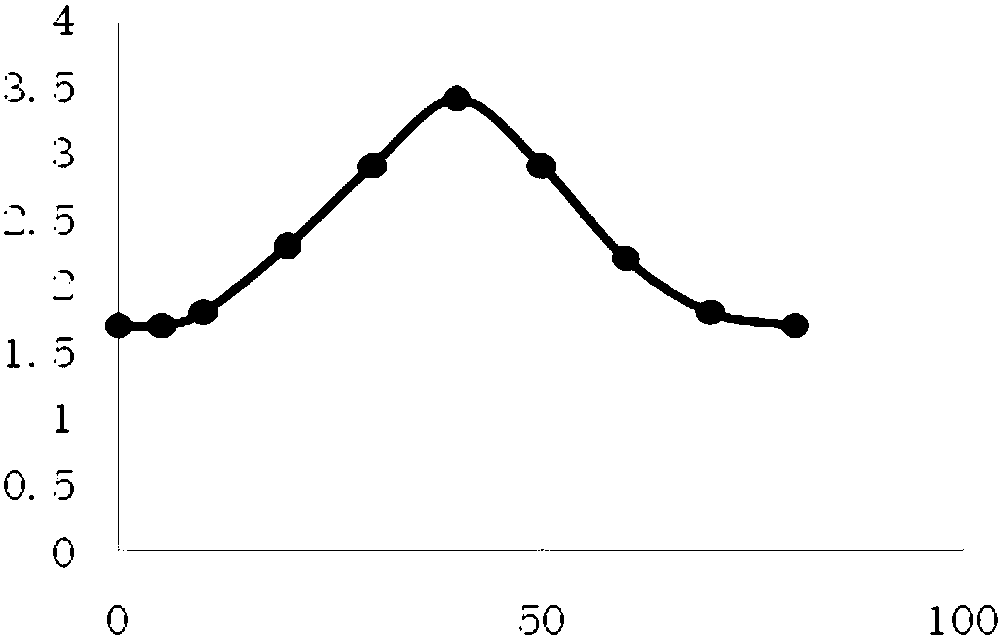
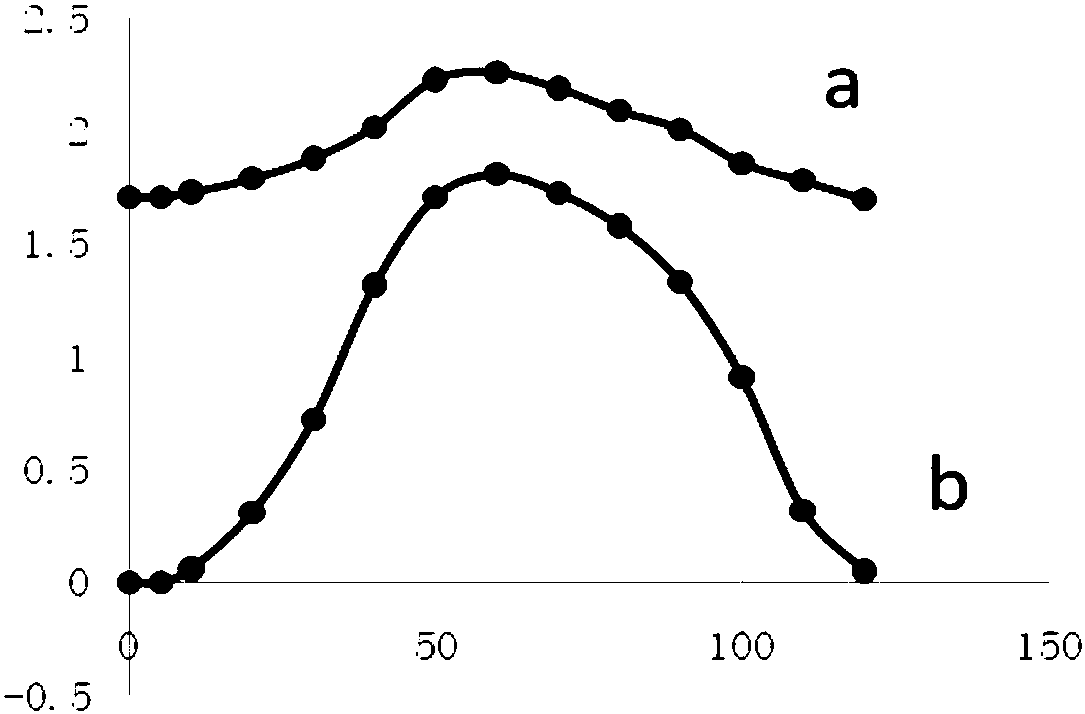
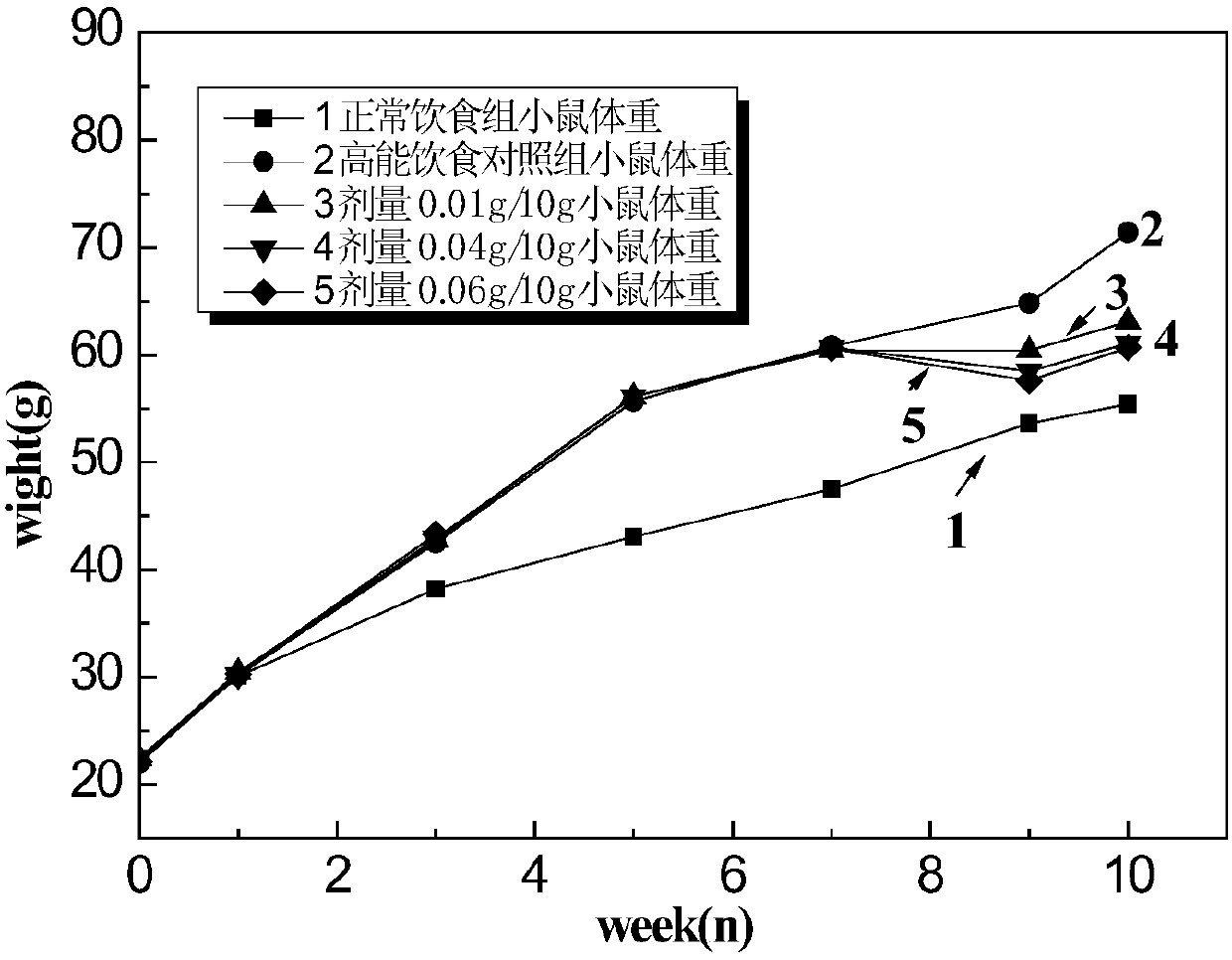
Beta-hydroxyl butyryl-amino acid compound, preparing method and application
A technology of hydroxybutyryl and amino acid, which is applied in the field of compounds, can solve the problems of different doses, different effects, low bioavailability, ketoacidosis in blood vessels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0122] β-Hydroxybutyryl-leucine pseudo-dipeptide
[0123] (1) Preparation of intermediate tert-butyldimethylsilyl ether β-hydroxybutyric acid
[0124] synthetic route:
[0125]
[0126]Ethyl β-hydroxybutyrate (132g, 1mol) and imidazole (136g, 2mol) were dissolved in 200mL of dichloromethane, cooled to 0-5°C, tert-butyldimethylsilyl chloride (195.8g, 1.3mol) was dissolved in 200 mL of dichloromethane was added dropwise into the reaction solution. After the dropwise addition, the ice bath was removed, and the reaction was carried out overnight at room temperature. After the gas chromatography monitors that the reaction is complete, add water to quench the reaction. After liquid separation, the organic phase is washed once with 50mL of 0.1M dilute hydrochloric acid and 50mL of saturated sodium chloride aqueous solution, and dried and concentrated to obtain tert-butyldimethylsilyl ether β-hydroxyl Crude ethyl butyrate. Add 2 times the volume of ethanol to the protected crude...
Embodiment A2
[0135] β-Hydroxybutyryl-phenylalanine pseudo-dipeptide
[0136]
[0137] The synthetic route can refer to the synthetic route of Example A1, which is prepared from the intermediate tert-butyldimethylsiloxane β-hydroxybutyric acid and ethyl phenylalanine hydrochloride, with a yield of 67.8%.
[0138] 1H NMR (400MHz, D2O) δ7.16(m,5H),4.40(m,1H),3.94(m,1H),3.12(m,1H),2.82(m,1H),2.19(m,2H) ,0.98(d,3H).
Embodiment A3
[0140] β-Hydroxybutyryl-isoleucine pseudo-dipeptide
[0141]
[0142] The synthetic route can refer to the synthetic route of Example A1, which is prepared from the intermediate tert-butyldimethylsilyl ether β-hydroxybutyric acid and isoleucine ethyl ester hydrochloride, with a yield of 62.8%.
[0143] 1H NMR (400MHz, D2O) δ4.11(m,1H),4.02(m,1H),2.34(m,2H),1.75(m,1H),1.32(m,1H),1.12(d,3H) ,1.05(m,1H),0.80(m,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com