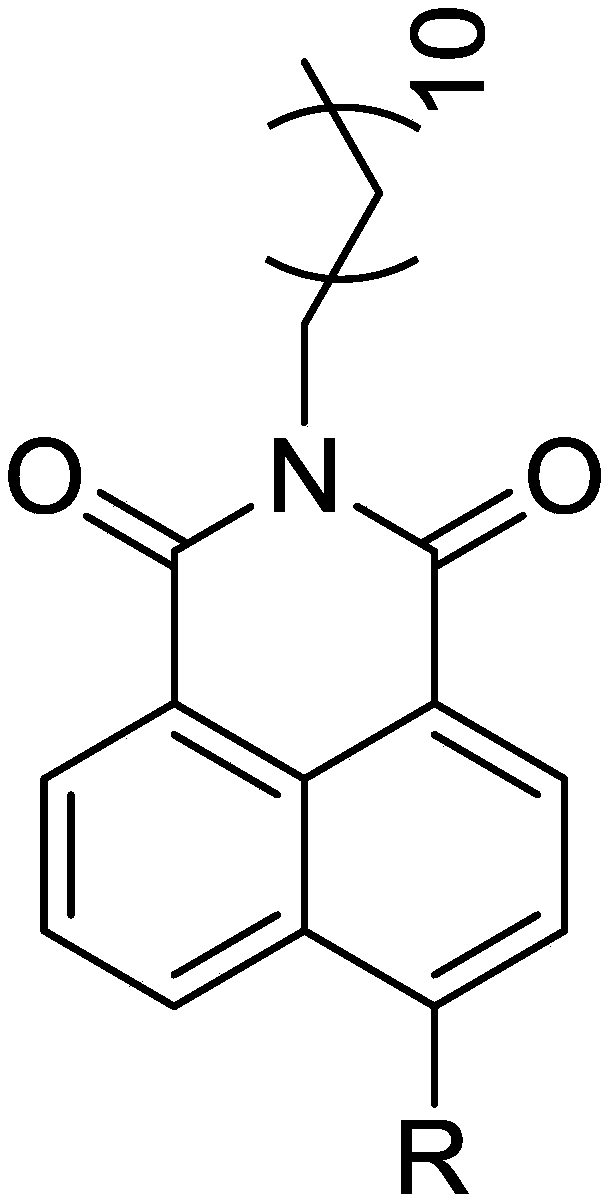
Fluorescent probes for labelling and/or detecting lipid droplets in cells as well as preparation and applications of fluorescent probes
A fluorescent probe and quasi-labeling technology, applied in the direction of fluorescence/phosphorescence, luminescent materials, measuring devices, etc., can solve the problems of unfavorable long-term fluorescence imaging analysis, easy fluorescence quenching, fluorescence instability, etc., and achieve convenient post-processing , good light stability, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
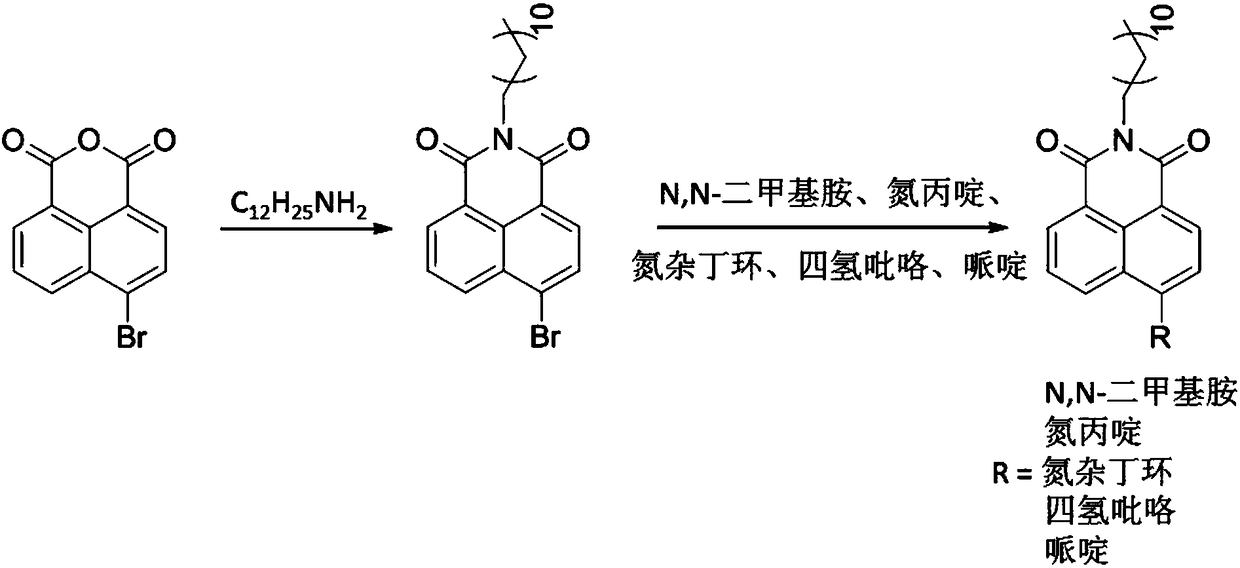
[0039] Example 1: The preparation of the fluorescent probe N-dodecyl-4-azetidine-1,8-naphthalimide, the basic synthesis process is as follows:
[0040] (1) Synthesis of N-dodecyl-4-bromo-1,8-naphthalimide:
[0041] In a 250mL single-necked bottle, 2.27g (10mmol) of 4-bromo-1,8-naphthalene anhydride and 2.78g (15mmol) of dodecylamine were dissolved in 100mL of ethanol, and refluxed at 90°C for 12 hours. After the heating was stopped, the precipitated solid was filtered and washed three times with 20 mL of ethanol to obtain 3.2 g of N-dodecyl-4-bromo-1,8-naphthalimide with a yield of 72%.
[0042] (2) Synthesis of N-dodecyl-4-azetidine-1,8-naphthalimide:
[0043] 0.44g (1mmol) N-dodecyl-4-bromo-1,8-naphthalimide, 0.1mL (1.5mmol) azetidine The alkane was dissolved, and reacted at 140° C. for 8 hours under nitrogen protection. After spin-drying the solvent, after separation by 200-300 mesh silica gel column chromatography at 25°C, spin-dry to obtain 280 mg of a red waxy solid t...
Embodiment 2
[0046] Embodiment 2: the mensuration of the ultraviolet-visible absorption spectrum of fluorescent probe:
[0047] The probe synthesized in Example 1 was prepared at a concentration of 10 μM with 20 mM HEPES solution (pH=7.4). Scan absorption in the 250nm-600nm band with a UV-visible spectrophotometer to obtain Figure 5 Mid-UV-Vis absorption spectrum.
Embodiment 3
[0048] Example 3: Determination of the fluorescence emission spectrum of the fluorescent probe.
[0049] The probe synthesized in Example 1 was prepared at a concentration of 10 μM with 20 mM HEPES solution (pH=7.4). Scanned with a fluorescence spectrometer under 450nm excitation Image 6Fluorescence emission spectra in .
[0050] Example 4 The fluorescent probe probe prepared in Example 1 labeled cells
[0051] Figure 7 The medium cell is colon cancer cell HT-29, and the probe used is synthesized in Example 1 at a concentration of 5 μM. HT-29 cells were inoculated in confocal small dishes at a concentration of 20,000 cells / dish, and cultured in 1640 medium for 3 days. Aspirate the medium, wash it twice with PBS buffer solution, add 2mL of fresh 1640 medium, and then add the probe solution synthesized in Example 1 at a concentration of 2mM, so that the concentration in the medium is 5μM, and in a CO2 incubator at 37°C After incubating for 10 min, the medium was sucked of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com