an oxygen candle
A technology of oxygen candle and candle body, applied in the field of high-efficiency oxygen release oxygen candle, can solve the problems of slow burning, easy moisture absorption of the formula, and affecting the oxygen production speed of the oxygen candle, so as to achieve reasonable formula, improve start-up reliability, and stable structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
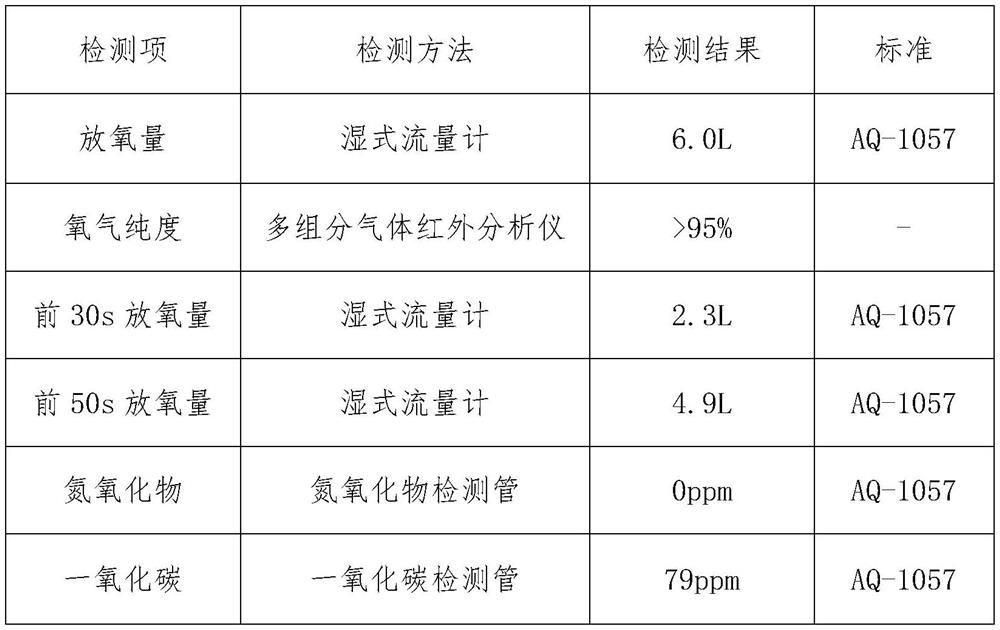
[0019] Absorb flammable layer 10g altogether, each component ratio is (mass percentage): chlorate 0.2%, magnesium powder 10%, barium chromate 85%, kaolin 4.8%;
[0020] The heating element layer is 52.1g, and the ratio is (mass percentage) 73% of sodium chlorate, 4% of tricobalt tetroxide, 6% of manganese dioxide, 5% of iron powder, 7% of cobalt powder, and 5% of kaolin.
[0021] Main candle body layer 300.05g, the ratio is (mass percentage) sodium chlorate 95%, potassium perchlorate 0.5%, tricobalt tetroxide 0.7%, manganese dioxide 0.8%, cobalt powder 1.0%, kaolin 2.0%. First, dry the chlorate in an infrared oven at 120°C for 30-60 minutes, then use a ball mill and a double-screw mixer to mix it evenly with other weighed materials, and add a certain amount of NaCrO with a concentration of 0.5-2%. 4 The aqueous solution is fully mixed, demolded and pressed into H=23mm oxygen-generating drug block; dried in an infrared drying oven at 120°C for 1 hour.
[0022] Experimental r...
Embodiment 2
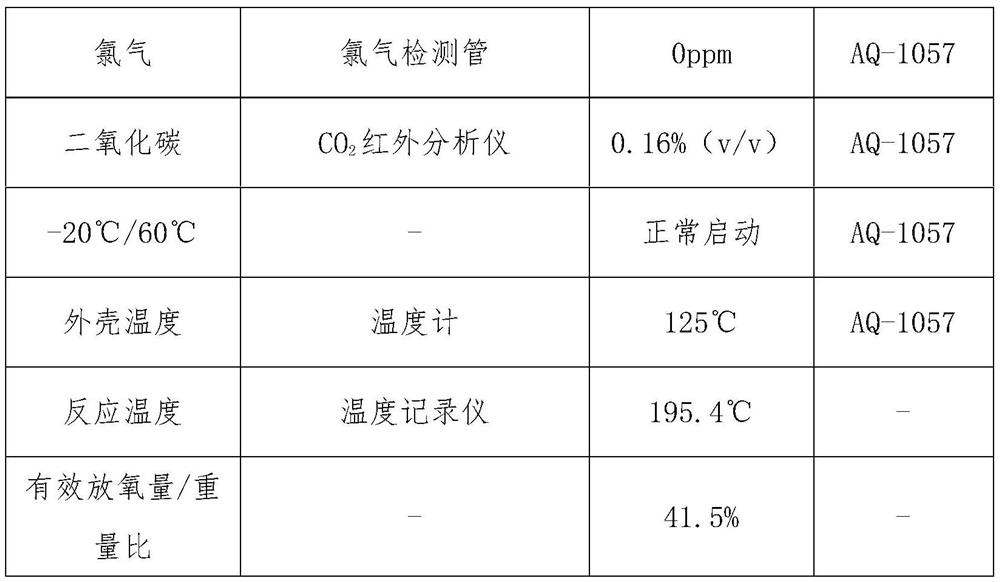
[0026] Absorb flammable layer 15g, the ratio is (mass percentage): chlorate 0.5%, magnesium powder 10%, barium chromate 84.5%, kaolin 5%;
[0027] The heating body layer is 59.12g, and the ratio is (mass percentage) 76% of sodium chlorate, 5% of tricobalt tetroxide, 5% of manganese dioxide, 3% of iron powder, 6% of cobalt powder, and 5% of kaolin.
[0028] Main candle body layer 285g, ratio is (mass percentage) sodium chlorate 95.5%, potassium perchlorate 0.7%, tricobalt tetroxide 0.7%, manganese dioxide 0.9%, cobalt powder 0.5%, kaolin 1.7%. First, dry the chlorate in an infrared oven at 120°C for 30-60 minutes, then use a ball mill and a double-screw mixer to mix it evenly with other weighed materials, and add a certain amount of NaCrO with a concentration of 0.5-2%. 4 The aqueous solution is fully mixed, demolded and pressed into H=23mm oxygen-generating drug block; dried in an infrared drying oven at 120°C for 1 hour.
[0029] Experimental results: the oxygen candle can...
Embodiment 3
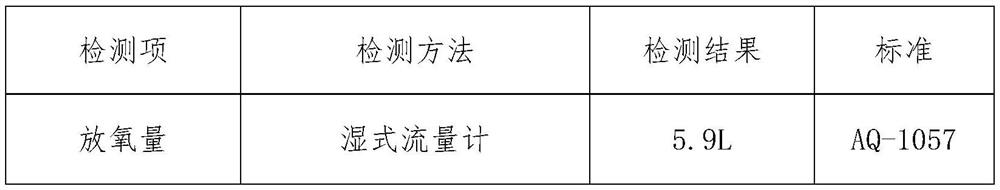
[0033] Absorb flammable layer 10g, the ratio is (mass percentage): zirconium powder 8%, barium chromate 83%, kaolin 9%;
[0034] The heating body layer is 48.5g, and the ratio is (mass percentage) 77% of sodium chlorate, 6% of tricobalt tetroxide, 5.5% of manganese dioxide, 4% of iron powder, 2.5% of magnesium powder, and 5% of kaolin.
[0035] The main candle body layer is 300.01g, and the ratio is (mass percentage) sodium chlorate 94.4%, potassium perchlorate 1.0%, tricobalt tetroxide 0.9%, manganese dioxide 1.2%, magnesium powder 1.0%, kaolin 1.4%. First, dry the chlorate in an infrared oven at 120°C for 30-60 minutes, then use a ball mill and a double-screw mixer to mix it evenly with other weighed materials, and add a certain amount of NaCrO with a concentration of 0.5-2%. 4 The aqueous solution is fully mixed, demolded and pressed into H=23mm oxygen-generating drug block; dried in an infrared drying oven at 120°C for 1 hour.
[0036] Experimental results: the oxygen c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com