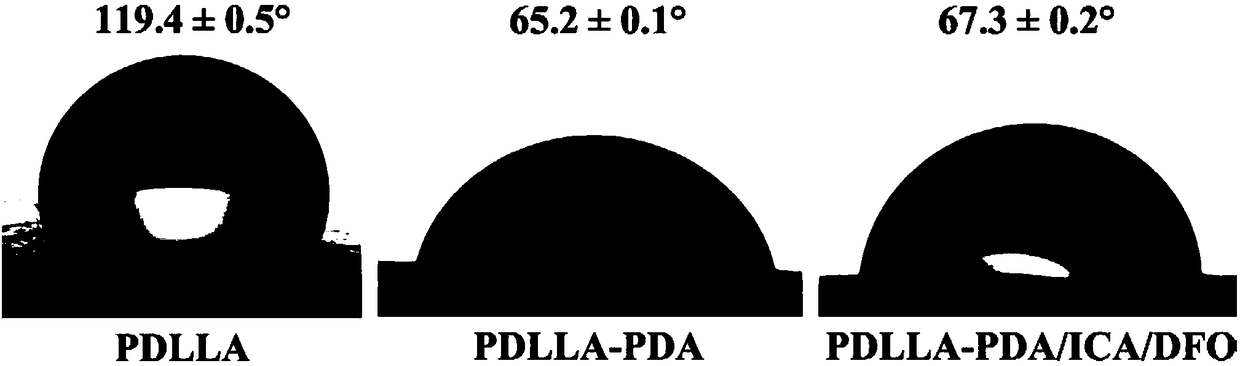
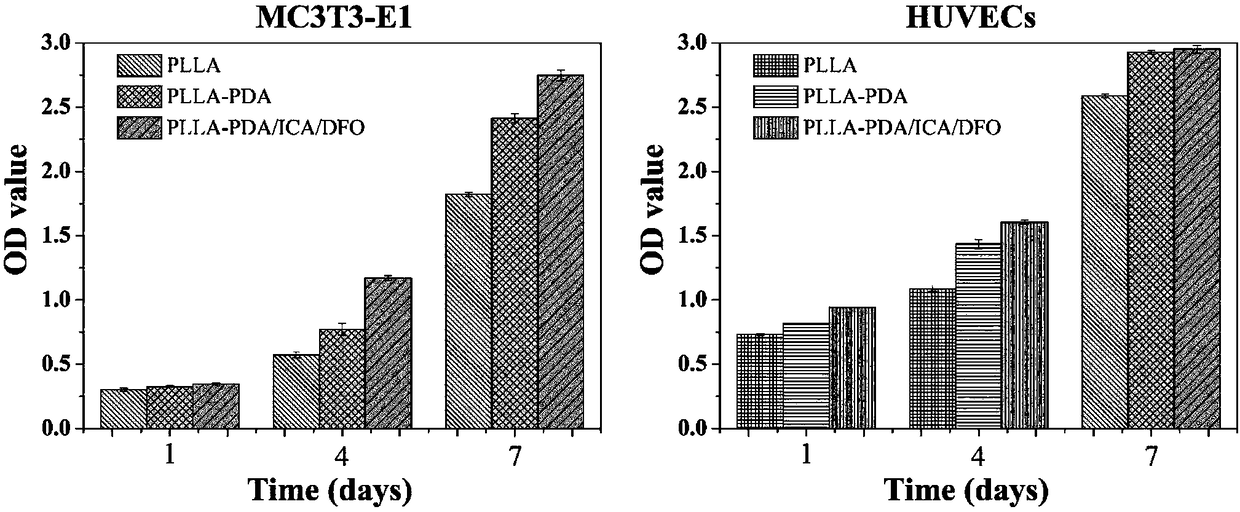
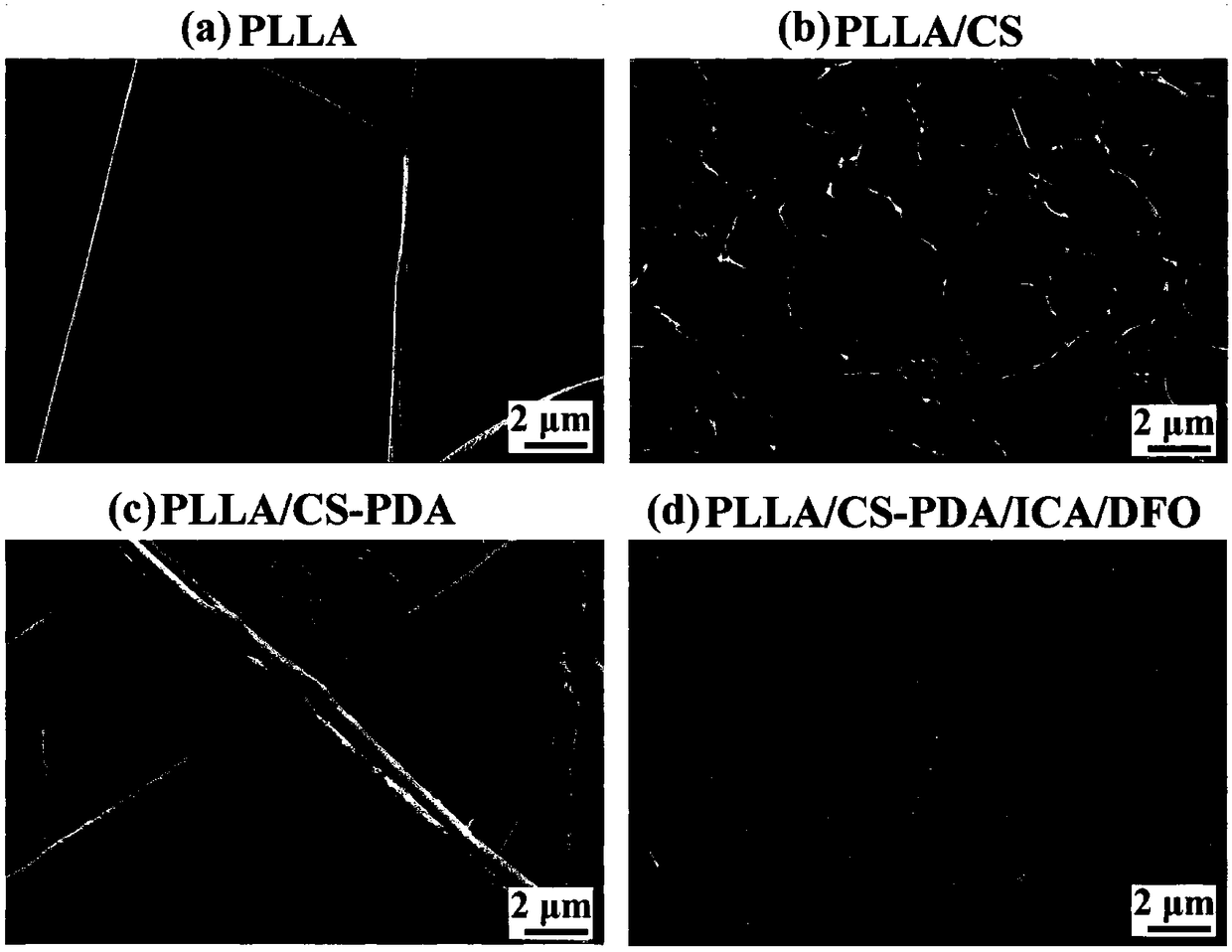
Polylactic-acid-based bone tissue support loading icariin and deferoxamine and preparation method and application of polylactic-acid-based bone tissue support
A technology of icariin and tissue scaffolds, which is applied in the field of polylactic acid-based bone tissue scaffolds and preparations, can solve the problems of lack of mechanical properties and single improvement, and achieve a composition and performance that are easy to control, low in cost, and easy to industrialize Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Prepare the PDLLA solution with chloroform to obtain an electrospinning solution with a concentration of 15% g / mL. After magnetic stirring and ultrasonic dispersion, the electrospinning solution is injected into the electrospinning solution supply device, and spinning is carried out under a static voltage of 20kV. The supply flow rate of the electrospinning solution is 2mL / h, and the distance between the receiving plate and the syringe pump needle is 15 cm to obtain PDLLA micron fiber scaffold.
[0043] (2) Dissolve trimethylaminomethane in deionized water, adjust the pH of the solution to 7 with 1mol / L hydrochloric acid while stirring, and prepare a 0.001mol / L trimethylaminomethane / hydrochloric acid buffer solution. Dopamine was dissolved in the above buffer solution, and a 1 g / L dopamine solution was obtained after magnetic stirring. Soak the PDLLA microfibrous scaffold in dopamine solution, react at room temperature and in the dark for 6 hours, then take out the...
Embodiment 2
[0047] (1) The PLLA solution was prepared using tetrahydrofuran solvent to obtain an electrospinning solution with a concentration of 25% g / mL. After magnetic stirring and ultrasonic dispersion, the electrospinning solution is injected into the electrospinning solution supply device, and spinning is carried out under a static voltage of 30kV. The supply flow rate of the electrospinning solution is 3mL / h, and the distance between the receiving plate and the syringe pump needle is 15 cm to obtain PLLA microfiber scaffolds.
[0048] (2) Dissolve trimethylaminomethane in deionized water, adjust the pH of the solution to 8 with 1mol / L hydrochloric acid while stirring, and prepare a 0.001mol / L trimethylaminomethane / hydrochloric acid buffer solution. Dopamine was dissolved in the above buffer solution, and a 2 g / L dopamine solution was obtained after magnetic stirring. The PLLA microfibrous scaffold was soaked in dopamine solution, reacted at room temperature and protected from ligh...
Embodiment 3
[0052] (1) The PLLA solution was prepared with toluene solvent to obtain an electrospinning solution with a concentration of 3% g / mL. After magnetic stirring and ultrasonic dispersion, the electrospinning solution was injected into the electrospinning solution supply device, and spinning was performed at a static voltage of 20 kV and a supply flow rate of 1 mL / h to obtain PLLA micron fiber scaffolds.
[0053] (2) Soak the PLLA microfibrous scaffold in a CS dilute acetic acid solution with a volume concentration of 5% acetic acid and a mass concentration of CS of 0.01%, for 12 hours. Then the scaffold was taken out and quenched at -140° C. for 1 h, and finally freeze-dried to obtain a PLLA / CS micro-nano composite fiber network scaffold with a hierarchical structure.
[0054] (3) Dissolve trimethylaminomethane in deionized water, adjust the pH of the solution to 8 with 1mol / L hydrochloric acid while stirring, and prepare a 0.001mol / L trimethylaminomethane / hydrochloric acid buffe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Fiber diameter | aaaaa | aaaaa |
| Aperture size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com