Single isomer of phosphoric acid/phosphonic acid derivative and medical application of single isomer
A technology of phosphonic acid derivatives, diastereomers, applied in the field of pharmaceutically acceptable salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
[0055] Example 1 9-[(R)-2-[[(R)-[[(S)-1-(isopropoxycarbonyl)-ethyl]-amino]-phenoxyphosphono]-methanol Preparation of Oxy]-Propyl]-Adenine (Ia-1-1)
[0056]
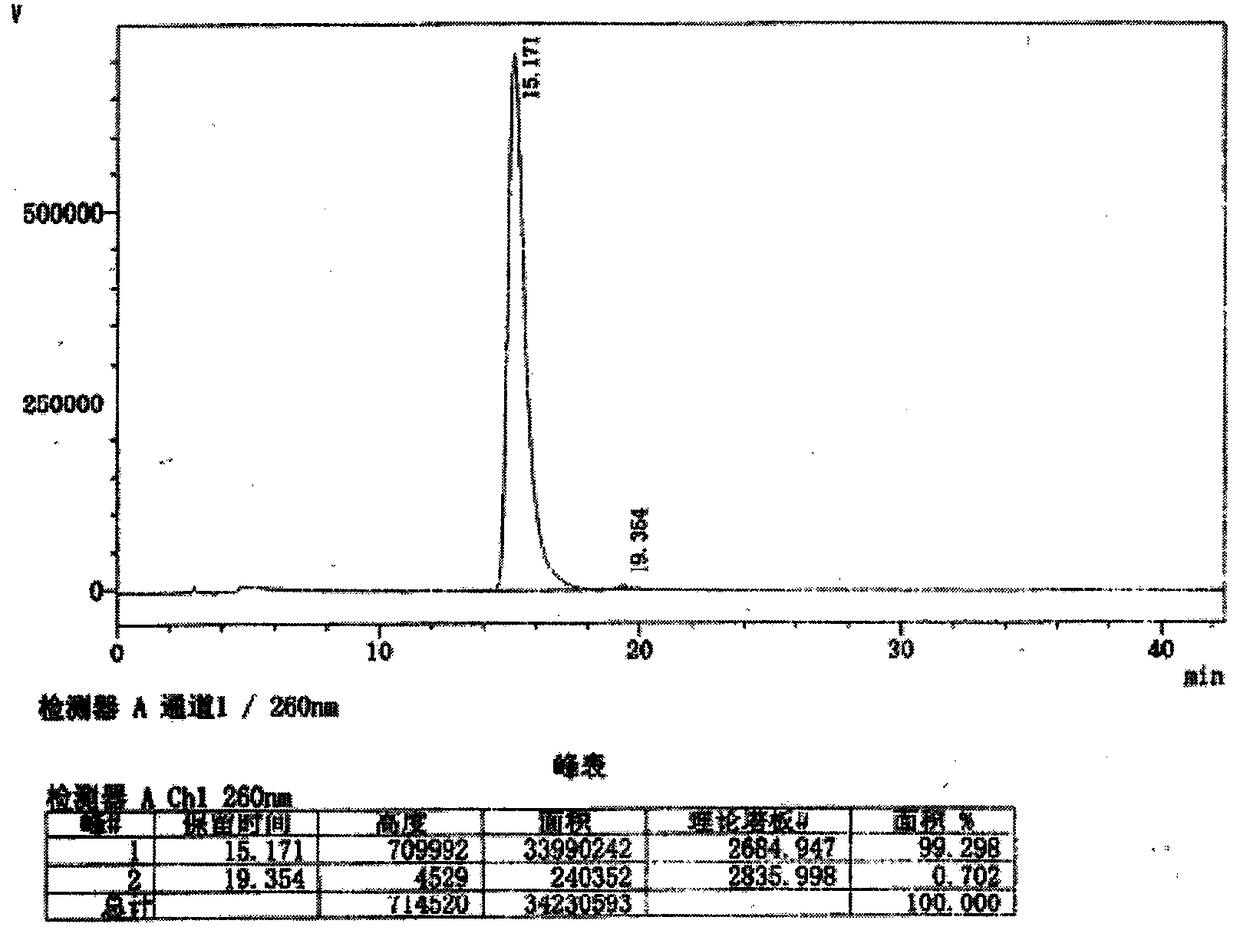
[0057] Add 14.6g 9-[(R)-2-(phosphonomethoxy)-propyl]-adenine (PMPA) and 9.6g phenol to 50ml N-methylpyrrolidone, heat to 85°C, add 6.3 ml triethylamine. Slowly add 13.4g of DCC under stirring, heat and stir overnight at 100°C, add 30ml of water after cooling. Set aside, filter off the solid, combine the washed filtrates, evaporate to dryness under reduced pressure, add 30ml of water, adjust the pH to 11 with 25% sodium hydroxide, filter off the solids, combine the washed filtrates, and extract with 30ml of ethyl acetate. The pH of the aqueous solution was adjusted to 3.1 with 37% hydrochloric acid, and a solid was precipitated upon standing. The solid was collected by filtration, added with 50ml of methanol, stirred and washed, filtered, and vacuum-dried to obtain 7.2g of phenol monoester derivative i of PMPA.
[0...
Embodiment 25
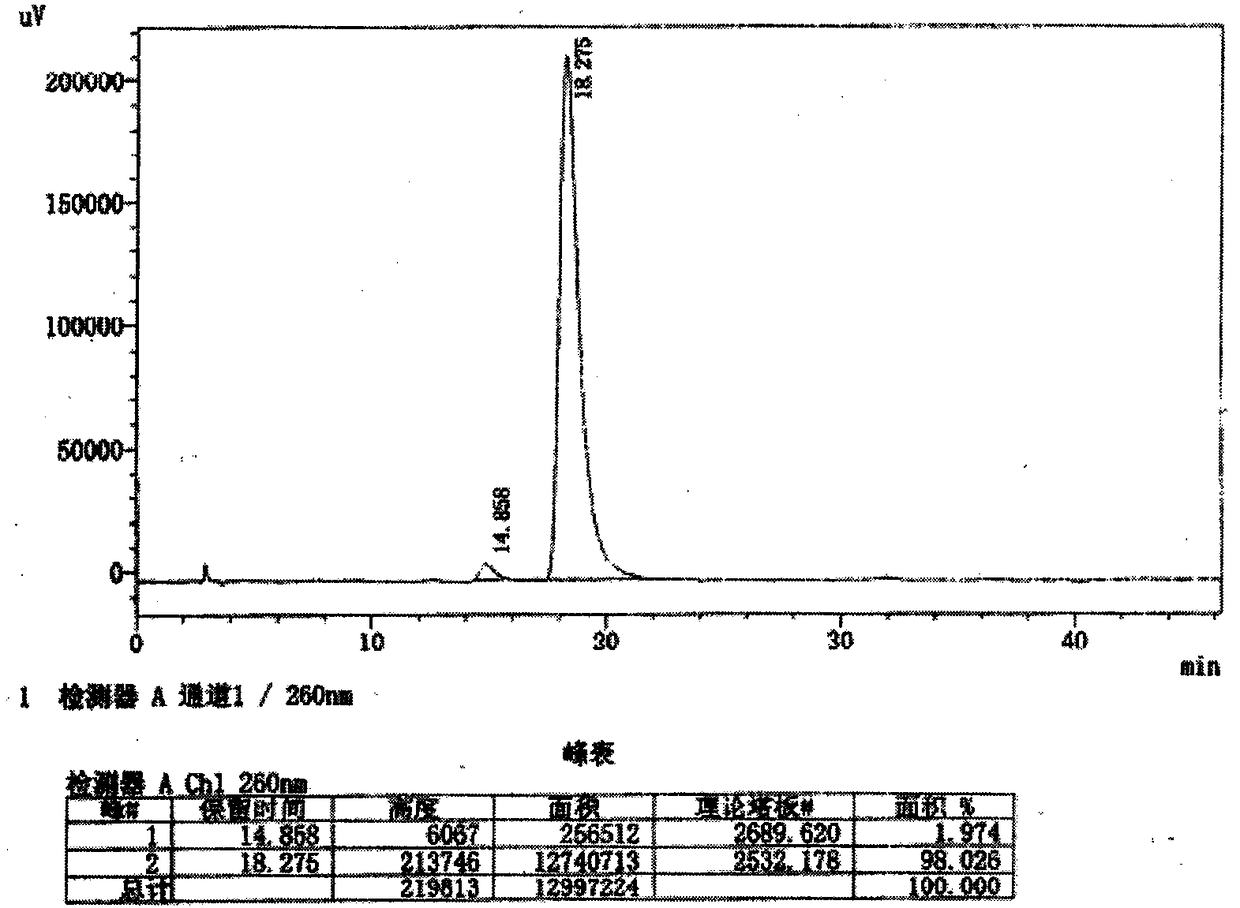
[0059] Example 2 5'-[(S)-[[(S)-1-(isopropoxycarbonyl)-ethyl]-amino]-phenoxyphosphoryl]-β-D-2'-deoxy - Preparation of 2'-a-fluoro-2'-β-C-methyluridine (Ia-2-1)
[0060] 2.1 Small-scale preparation of (S)-2-[(R)-(2-chloro-4-nitro-phenoxy)-phenoxy-phosphorylamino]propionic acid isopropyl ester (vi-b)
[0061] Dissolve 10 g (47.3 mmol) of phenyl dichlorophosphate in 50 mL of dry dichloromethane, cool to 0°C; add 7.94 g (47.3 mmol) of L-alanine isopropyl hydrochloride (iii-1) Solid, cooled to -78°C. A solution of 13.8ml of triethylamine (94.6mmol) dissolved in 50mL of dry dichloromethane was added dropwise with stirring, and the rate of addition was controlled to keep the reaction temperature at -78°C. Stir for 30 minutes after the addition, and then warm up to 0°C; within 5-10 minutes, add 8.2 grams (47.3 mmol) of 2-chloro-4-nitro-phenol and 6.6 ml (47.3 mmol) of triethylamine dropwise under stirring Dissolve in 20 mL of dry dichloromethane; keep at 0°C and continue stirring fo...
Embodiment 3
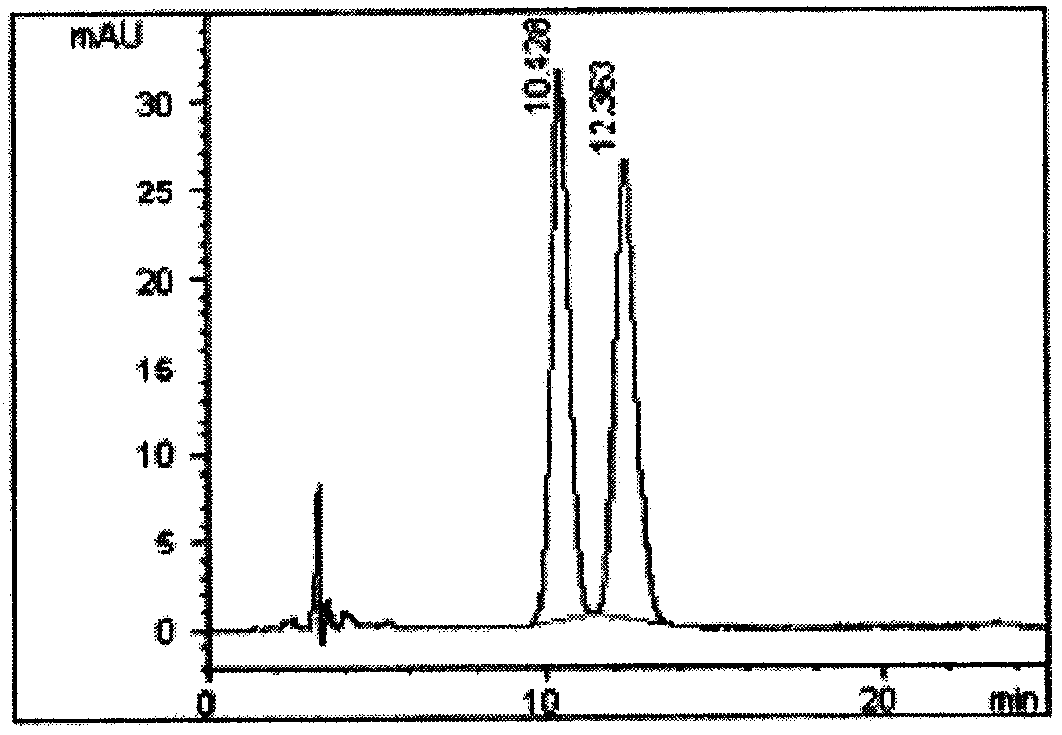
[0067] Example 3 Subacute Toxicity Evaluation and Tissue Distribution Evaluation of Continuous Administration of Tenofovir Alafenamide (TAF) and Ia-1-1 Beagle Dogs
[0068] Male Beagle dogs (7-8 months old, weighing 7-10kg) were randomly divided into groups of 4 and weighed. Carboxymethylcellulose sodium suspension containing 30 mg / kg TAF (purchased from Shanghai Hanxiang Biotechnology Co., Ltd., HPLC content 98%) or Ia-1-1 was given by intragastric administration once a day for 14 consecutive days. After the last administration, fasting without food and water for 4 hours, taking blood from vein to prepare serum for determination of blood biochemical indexes. Then, they were executed, and the organs and tissues were taken and divided into two parts. Take a part of the tissue, weigh it, add pure water to a homogenizer to make a 10% homogenate, and use high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS) to determine the active form of tenofovir in each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com