A 3- 18 f-Fluorolactic acid analogue and its preparation method and application
An analogue, lactic acid technology, applied in the fields of medicinal chemistry and nuclear medicine, can solve the problems of limitation, unsuitable molecular probe production, short half-life, etc., and achieve the effect of stable labeling rate, fast blood clearance rate, and good biodistribution characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、3-18
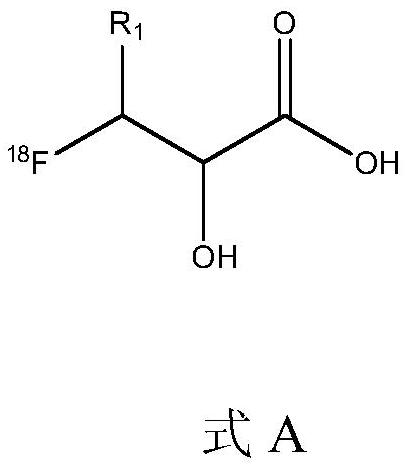
[0034] Embodiment 1, 3-[18F] fluoro-2-hydroxyl-propionic acid (R in formula A 1 for the synthesis of hydrogen)
[0035] Use 1ml of acetonitrile aqueous solution containing 3.6mg of cesium carbonate and 40mg of amino polyether K222 to elute 18F- into the reaction flask, evaporate to dryness at 110°C under negative pressure, add 1.0mL of anhydrous acetonitrile and evaporate to dryness. 20 mg of the precursor compound methyl 2,3-epoxy propionate (in the formula B, R 1 for hydrogen, R 2 (methyl) was dissolved in 1 mL of anhydrous tert-amyl alcohol, added to the reaction bottle, sealed and heated, and reacted at 120°C for 20 min. After the reaction is finished, cool to room temperature, inject into a reversed-phase C18 semi-preparative column (5 μm, 250×10 mm, product of YMC Company, Japan), and collect the component with a retention time of 7.5 to 9.0 min, which is the fluorine-18 labeled intermediate, namely the formula C The indicated intermediate, R 1 for hydrogen, R2 is me...
Embodiment 2、3-18
[0036] Example 2, Biodistribution test of 3-[18F]fluoro-2-hydroxyl-propionic acid
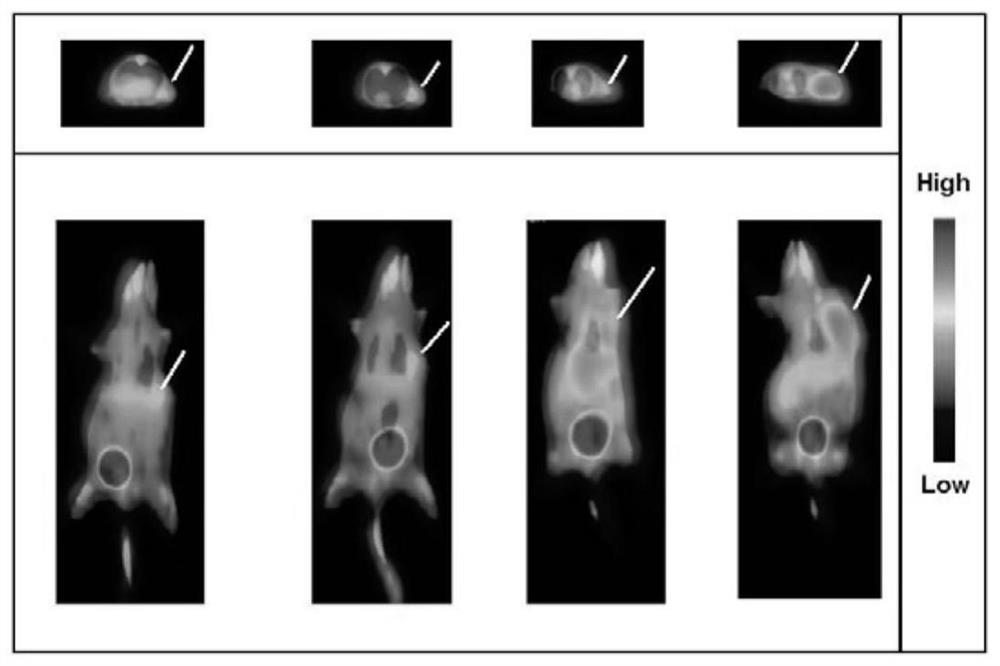
[0037] Preparation of tumor mouse model: After diluting the S180 ascites cells taken from the peritoneal cavity of the mouse by 2 times with normal saline, inject 0.2ml subcutaneously in the right lower limb of the ICR mouse, a solid tumor can be formed in 7 days, and then the compound can be used for biological evaluation and evaluation. Used in Micro-PET imaging experiments.
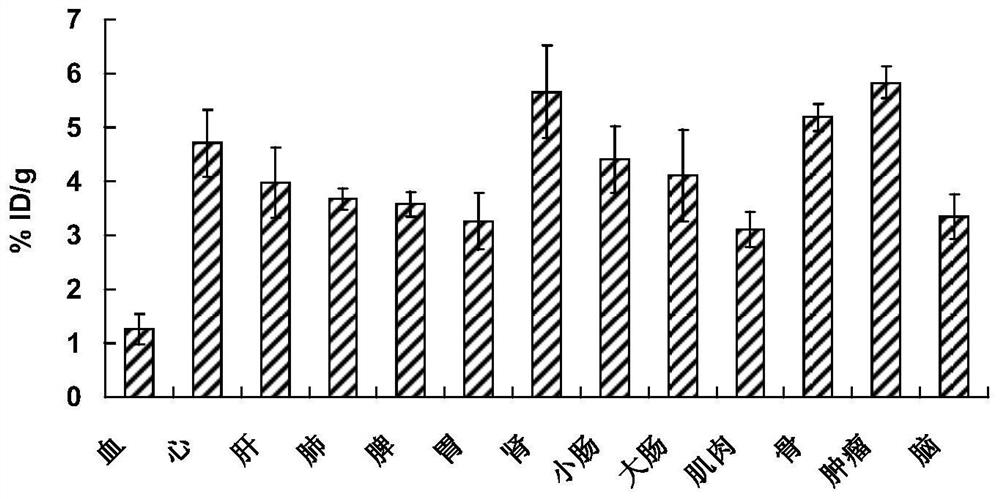
[0038] To 4 S180 tumor model ICR mice, 74kBq of 3-[18F]fluoro-2-hydroxy-propionic acid injection prepared in Example 1 were injected into the tail vein respectively, PET imaging was performed at 60 minutes, and they were executed after the imaging was completed. Dissect and take the organs and tissues of interest: blood, heart, liver, lung, kidney, stomach, large intestine, small intestine, bone, muscle, tumor, brain, etc., weigh and count them respectively, and perform decay correction on each tissue sample The counts...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com