A kind of ionic liquid-based electrolyte for lithium-air battery and its lithium-air battery system
A lithium-air battery and ionic liquid technology, which is applied to battery electrodes, fuel cell half-cells, secondary battery-type half-cells, secondary batteries, etc., can solve the problems of poor electrode wettability and reduce interface impedance , High electrochemical stability, low viscosity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 2.4mL N‐methyl‐N‐propylpyrrole bistrifluoromethanesulfonimide salt (PYR 1(1o2) TFSI) and 3.6mL dimethyl sulfoxide (DMSO), the volume ratio is 0.4:0.6 (where x=0.4), the two are mixed, and 0.9115g lithium hexafluorophosphate LiPF is added 6 (1mol / L), then add 0.0827g lithium nitrate (0.2mol / L), and magnetically stir for 24h to obtain an ionic liquid-based mixed electrolyte. The above steps are all operated in a glove box with water content and oxygen content less than 0.5ppm. Among them, the ionic liquid cationic PYR 1(1o2) + The structural formula is:
[0026] (where R1 is CH 2 CH 2 OCH 3 , R2 is CH 3 ),
[0027] Moreover, the anion of the ionic liquid is (CF 3 SO 2 ) 2 N - , abbreviated as TFSI - .
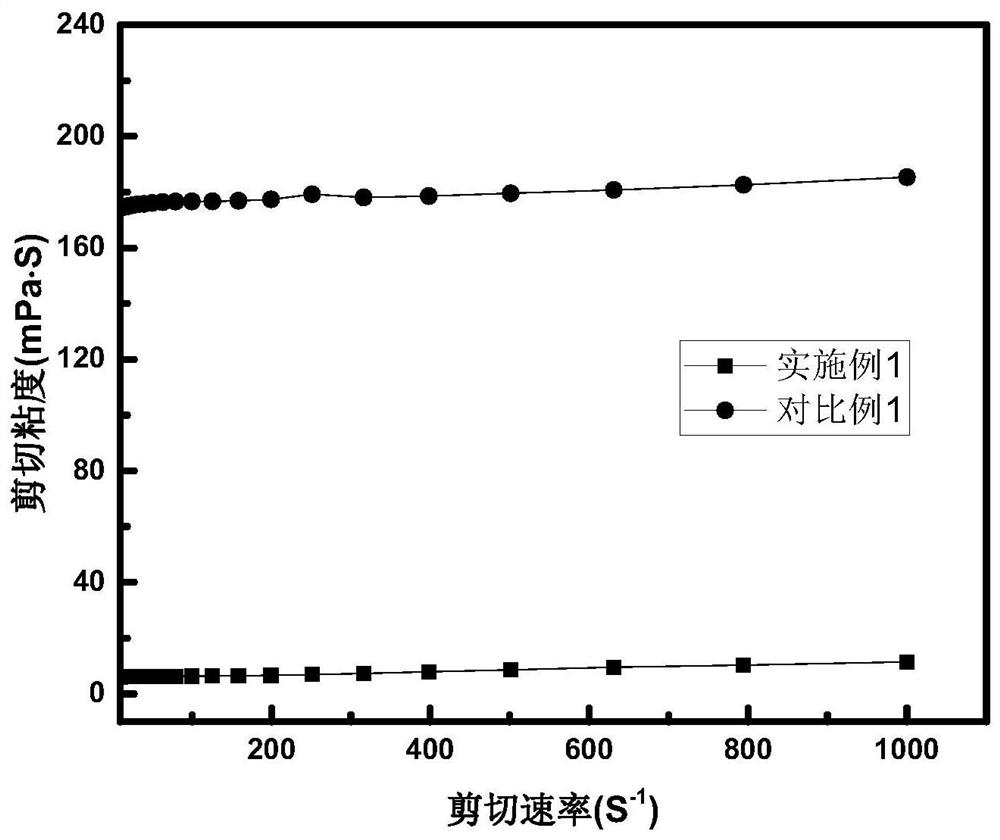
[0028]figure 1 Be the variation curve graph of the shear viscosity of embodiment 1 with shear rate. The shear viscosity of the mixed electrolyte is small, when the shear rate is 100S -1 , the shear viscosity of the ionic liquid-based electrolyte is 6...
Embodiment 2
[0032] Measure 1.8mL of N-methyl-N-butylpyrrole trifluoromethanesulfonate (PYR 14 CF 3 SO 3 ) and 4.2mL tetraethylene glycol dimethyl ether (TEGDME), the volume ratio is 0.3:0.7 (where x=0.3), the two are mixed, and 1.3781g lithium bistrifluoromethylsulfonimide LiTFSI (0.8mol / L) dissolved in it, then add 0.2069g lithium nitrate LiNO 3 (0.5mol / L) into the mixed solvent, and magnetically stirred for 24h to obtain an ionic liquid-based mixed electrolyte. The above steps are all operated in a glove box with water content and oxygen content less than 0.5ppm. Among them, the ionic liquid cationic PYR 14 + The structural formula is:
[0033] (where R1 is CH 2 CH 2 CH 2 CH 3 , R2 is CH 3 ),
[0034] Moreover, the anion of the ionic liquid is CF 3 SO 3 - .
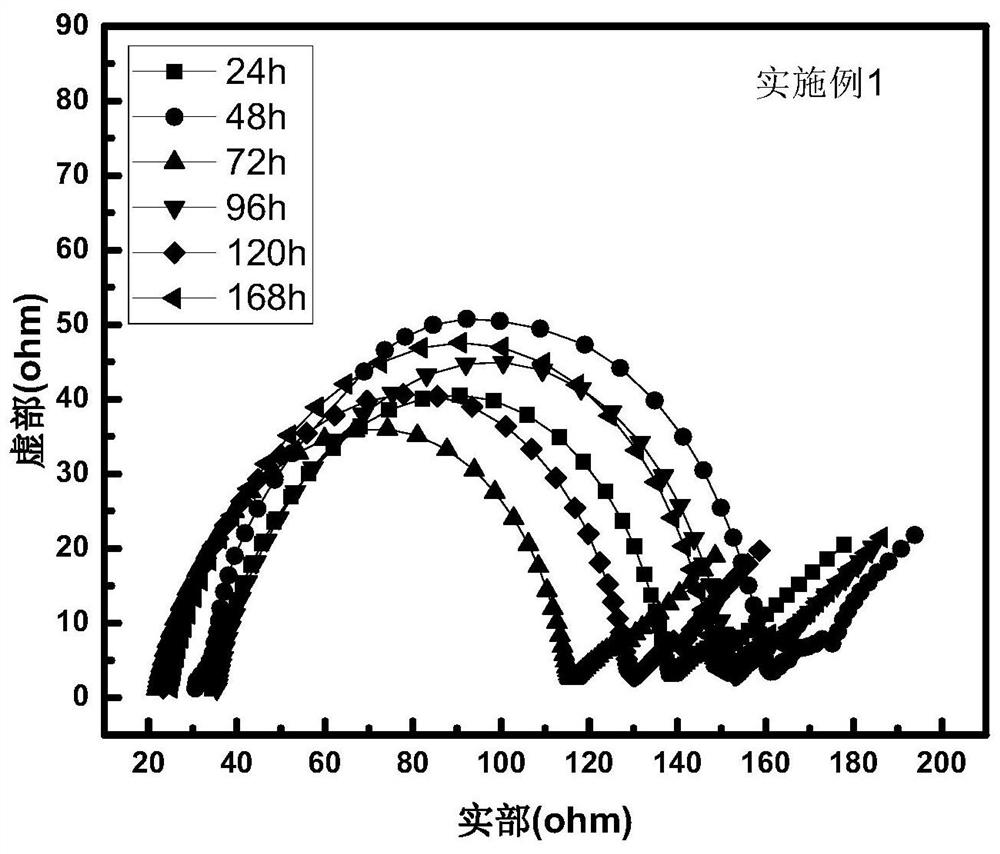
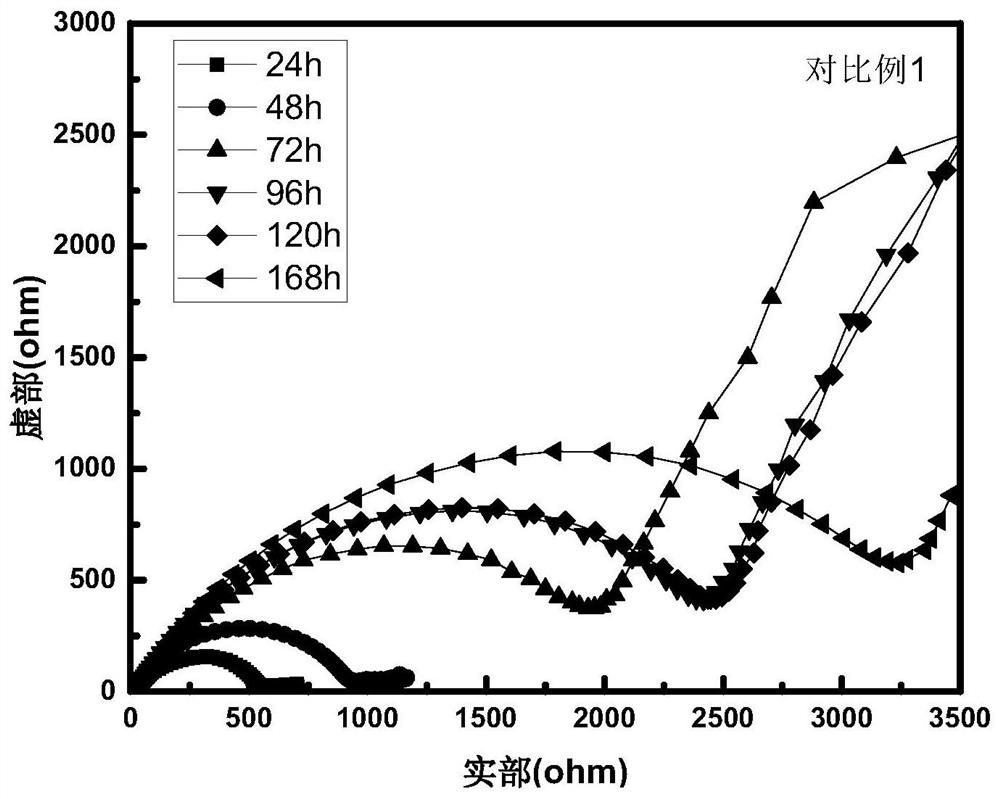
[0035] figure 2 The Li / / electrolyte / / Li battery assembled from the electrolyte prepared in Example 2 and the metal lithium sheet was tested for the change of the AC impedance of the battery over time. The inte...
Embodiment 3
[0039] Take 1.2mL of N‐methyl‐N‐butylpyrrole trifluoromethanesulfonate (PYR 13 CF 3 SO 3 ) and 4.8mL sulfolane (SL), the volume ratio is 0.2:0.8 (wherein x=0.2), the two are mixed, and 0.9361g lithium trifluoromethanesulfonate LiCF 3 SO 3 (1mol / L) was dissolved in the mixed solution, and then 0.2482g lithium nitrate LiNO 3 (0.6mol / L) into the mixed solution, and magnetically stirred for 24h to obtain an ionic liquid-based electrolyte. The above steps are all operated in a glove box with water content and oxygen content less than 0.5ppm. Among them, the ionic liquid cationic PYR 13 + The structural formula is:
[0040] (Wherein, R1 is CH2CH2CH3, R2 is CH3),
[0041] Moreover, the anion of the ionic liquid is CF 3 SO 3 - .
[0042] The above ionic liquid-based electrolyte is used to assemble a lithium-air battery, while the positive electrode material is carbon nanotube in-situ composite birnessite-type manganese dioxide, and the negative electrode uses metallic li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear viscosity | aaaaa | aaaaa |
| shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com