Controlled-release antibacterial dressing and preparation method thereof
A technology of antibacterial agent and antibacterial composition, applied in the field of slow-release antibacterial dressing and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: Preparation of drug-loaded starch microsphere antibacterial dressing
[0095] (1) Preparation of starch microspheres embedded with polyhexamethylbiguanide hydrochloride (PHMB)
[0096] Pour 50 mL of mixed solution of cyclohexane and chloroform (V (cyclohexane): V (chloroform) = 4: 1) into a 250 mL three-necked flask as the oil phase, and add 2 g of emulsifier (where the emulsifier The mass ratio of Span60:Tween60=3:2), heating and stirring at 50° C. to completely dissolve the emulsifier, and set aside.
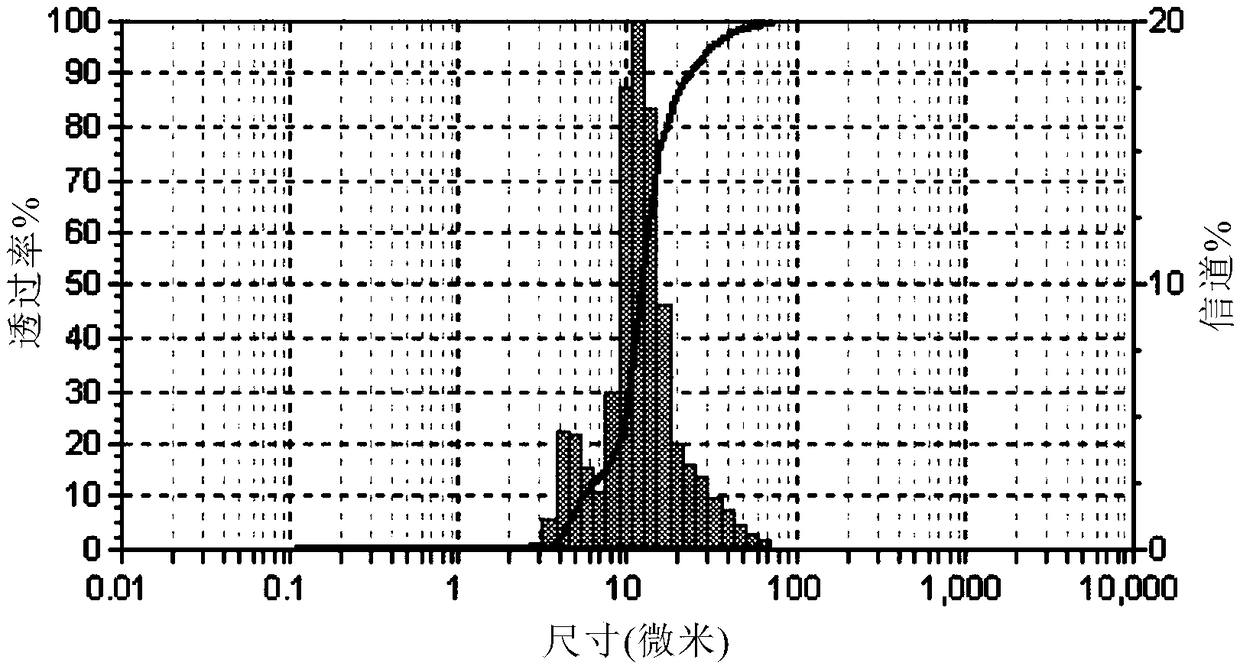
[0097] Weigh 1.5 g of soluble starch into 15 mL of distilled water, heat and dissolve to prepare starch=10wt% aqueous solution. Measure 10mL of the starch aqueous solution and add 0.2g MBAA (N,N'-methylenebisacrylamide), fully dissolve and add dropwise to the above oil phase, keep stirring at 500r / min, and take samples under an optical microscope in due course Observe the shape of the emulsion. After formation of uniform droplets, 0.1 g of ceric ammoniu...
Embodiment 2
[0105] Embodiment 2: the antibacterial effect of drug-loaded starch microsphere antibacterial dressing on Escherichia coli E.C (ATCC25922) and Staphylococcus aureus S.a (ATCC6538)
[0106] (1) Antibacterial test
[0107] Cut the sample into a size of 1.0cm×1.0cm, put it into a 250ml Erlenmeyer flask, add 70ml of PBS and 5ml of bacterial suspension, and adjust the concentration of bacterial suspension in PBS to 1×10 4 -9×10 4 CFU / ml, fix the Erlenmeyer flask on a vibrating shaker, shake it at 150r / min for 24h, take the sample solutions of the experimental group (with sample) and the blank group (without sample), respectively, dilute with PBS appropriately, and pour it into agar Plates were inoculated for colony counting. At the same time, a control group should be set up, and only PBS and bacterial suspension should be added to the control group. The antibacterial rate is the ratio (in percentage) of the difference between the average number of colonies before and after shak...
Embodiment 3
[0112]The long-acting antibacterial test of embodiment 3 drug-loaded starch microsphere antibacterial dressings to E.C and S.a
[0113] Test method is with reference to embodiment 2, and drug-loaded starch microsphere antibacterial dressing is to E.C and S.a long-acting antibacterial test, and drug-loaded starch microsphere composition is to E.C and S.a antibacterial action test result as shown in table 2 and table 3:
[0114] "+" indicates that it has antibacterial effect on bacteria,
[0115] "-" indicates that there is basically no antibacterial effect or weak antibacterial effect on bacteria.
[0116] Table 2 Long-acting antibacterial test of drug-loaded starch microsphere antibacterial dressings on E.C and S.a
[0117] value
symbol
>80
+++
60
++
50
+
40
―
30
――
20
―――
[0118] The antibacterial ef...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com