Application of a Class of Dianilino-Biphenyl Carbonyl Compounds in Photocuring Formula System
A compound and light-curing technology, which is applied in the field of diphenylamino biphenyl carbonyl compounds and radiation-cured formula products, can solve the problems of high energy consumption and short life, mercury vapor does not conform to new strategic trends, etc., and achieve great development significance. The effect of high monomer conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: DPA-BPHO is synthesized according to the following route
[0021]
[0022] (a): caproyl chloride, aluminum trichloride, anhydrous dichloromethane;
[0023] (b): Potassium carbonate, tetrakis(triphenylphosphine) palladium, toluene / ethanol / water, 90°C, 12h;
[0024] 1. Synthesis of p-1-p-bromophenylhexanone
[0025] Quickly put aluminum trichloride (5.32g, 0.04mol) in a dried 250mL three-neck flask, vacuumize and fill with nitrogen several times, then weigh bromobenzene (3.14g, 0.02mol) and inject it, cool to 0°C, Add hexanoyl chloride (4.03g, 0.03mol) slowly, and after the dropwise addition is complete, remove the ice bath, stir at room temperature for 5h, and monitor the reaction by TLC until the end. The system was then poured into ice water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated to obtain 3.0 g of a white flaky solid.
[0026] 1 H NMR (400MHz, CDCl 3 )δ7.82(d, J=8.6Hz, 2H), 7.59(d, J=8.6Hz, 2H), 2.93(dd,...
Embodiment 2
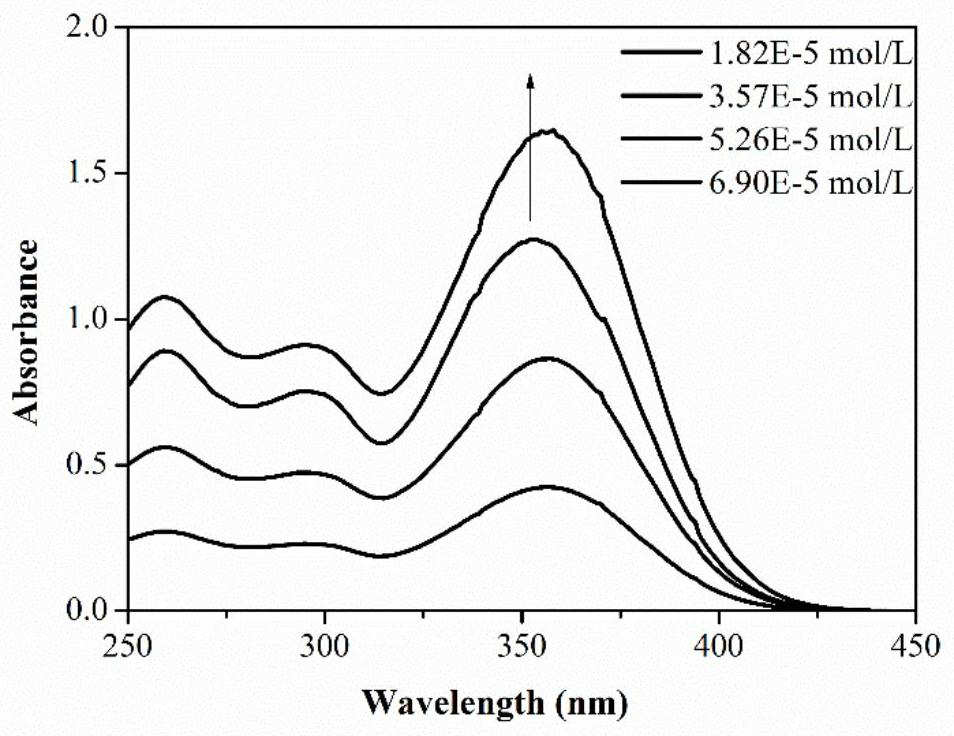
[0030] Embodiment two: the photochemical physical property of molecule
[0031] Molecular DPA-BPHO in embodiment one has carried out ultraviolet-visible light spectral analysis, as figure 1 It is the ultraviolet spectrogram of the photoinitiator molecule at different concentrations. According to the Lambert-Beer law and the linear fitting of the relationship between the absorbance and the concentration in the figure, the molar extinction coefficient of the target product at different wavelengths can be calculated, as shown in the following table :
[0032]
Embodiment 3
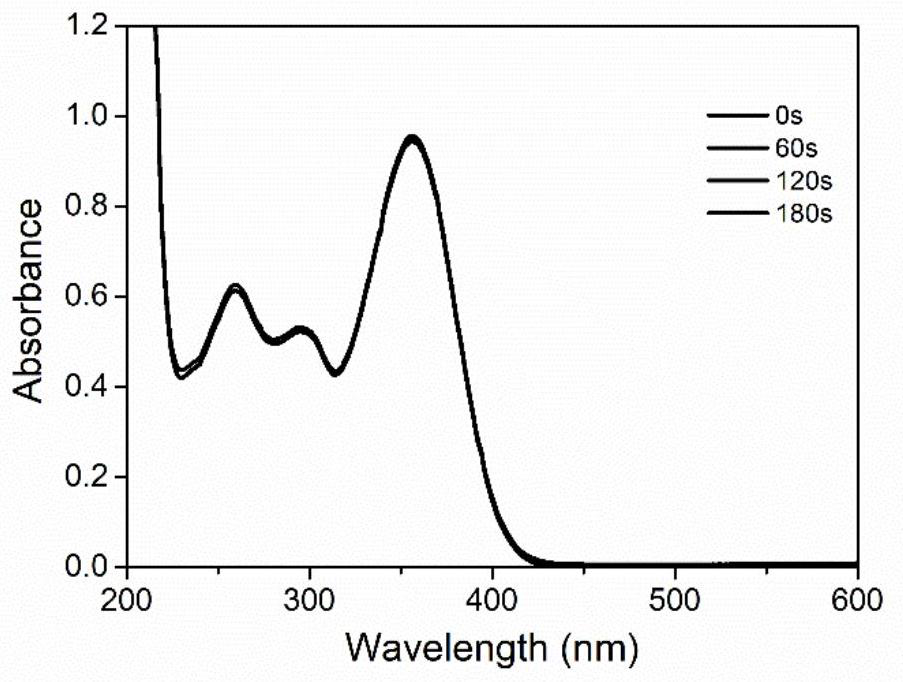
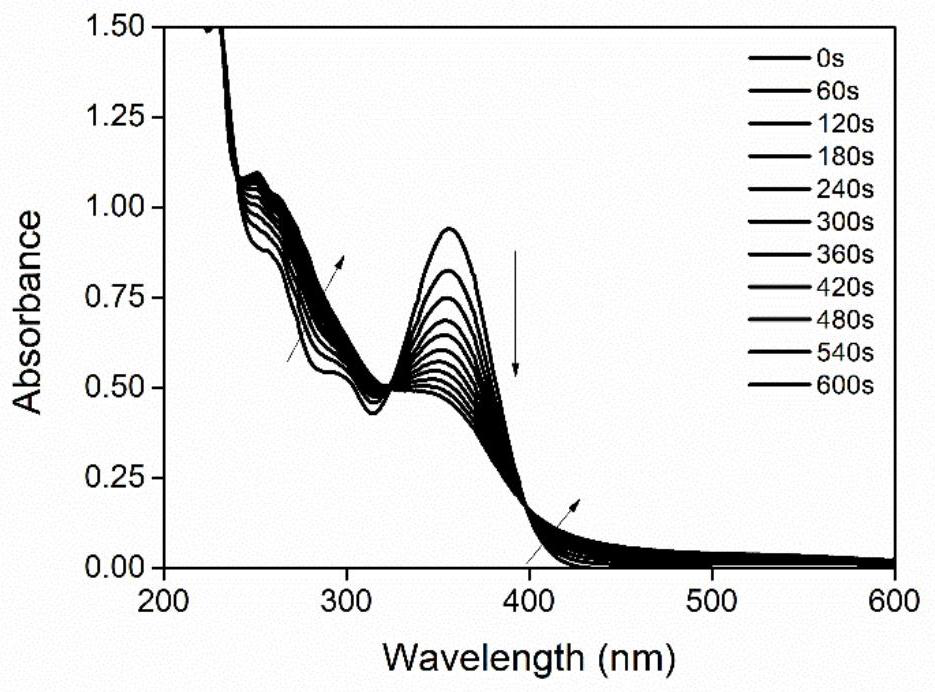
[0033] Example three: photodegradation properties of molecules
[0034] The molecule in Example 1 is illuminated, and the real-time spectrum change is determined by ultraviolet analysis, and the acetonitrile solution of a certain concentration of DPA-BPHO placed in a 3ml ultraviolet cuvette is irradiated with a 385nm LED light source, and then the length of illumination is compared to the spectrum. influence, such as figure 2 shown. It is found that the target molecule does not undergo photolysis basically under light irradiation, so adding 2 equivalents of iodonium salt solution, repeating the above experiment, it is found that the molecule undergoes obvious photodegradation, such as image 3 As shown, it is speculated that DPA-BPHO can act as a sensitizer of diphenyliodonium salt to initiate cationic polymerization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com