Dynamically-repairable polyurethane based on oxime and preparation method thereof
A technology of polyurethane and agglomerate oxime, applied in the field of polyurethane materials, can solve the problems of lack of recycling and reprocessing, unfavorable environmental protection and energy saving, and no reports on polyurethane.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1 bifunctional oxime B-(S-R) 2 preparation of
[0087] a) p-hydroxyacetophenone (compound shown in formula (II), wherein, R 1 is phenylene, R 2 Methyl) (52.37g) was dissolved in tetrahydrofuran solution (300mL), then allyl bromide (compound shown in formula (III), wherein, m is 1, X is Br) (37.3mL) and sodium carbonate (79g), reacted at 30°C for 3h; the reaction system was concentrated, diluted with water (200mL), extracted with ethyl acetate (3×200mL), concentrated, and dried over anhydrous sodium sulfate to prepare the intermediate product a;
[0088] b) The above intermediate product a was dissolved in 270mL H 2 O / EtOH (1:1, v / v), and add hydroxylamine hydrochloride (40.1g) and sodium carbonate (78.9g), react at 40 ℃ for 5h; Ethanol was washed three times to obtain a white solid, which was a compound (71.36g) shown in formula (IV), wherein, R 1 is phenylene, R 2 is methyl, m is 1; the two-step yield of the compound is 97%.
[0089] The structural c...
Embodiment 2 3
[0097] Example 2 Trifunctional oxime B-(S-R) 3 preparation of
[0098] The compound shown in formula (IV) (wherein, R 1 is phenylene, R 2 is methyl, m is 1; 13.22g) was dissolved in 100mL ethyl acetate, and 1,1,1-trimethylolpropane tris(3-mercaptopropionate) (9.67g) and 0.01 equivalents (for alkenes) were added Benzoin dimethyl ether (0.09g) was reacted for 3h under ultraviolet light irradiation; the solvent was concentrated and removed to obtain the trifunctional oxime B-(S-R) 3 , the specific structure of the prepared multifunctional oxime is shown in the following formula (IX):
[0099]
[0100] The structural confirmation data are as follows:
[0101] 1 H NMR (300MHz, CDCl 3 , ppm) δ9.10 (br s, 3H), 7.56 (d, J = 11.6Hz, 6H), 6.88 (d, J = 9Hz, 6H), 4.06-4.03 (m, 9H), 2.79 (t, J =7.2Hz,6H),2.72(t,J=7.2Hz,6H),2.64(t,J=6.9Hz,6H),2.27(s,9H),2.05(m,6H),1.49(q,J =7.5Hz, 2H), 0.88(t, J=7.5Hz).
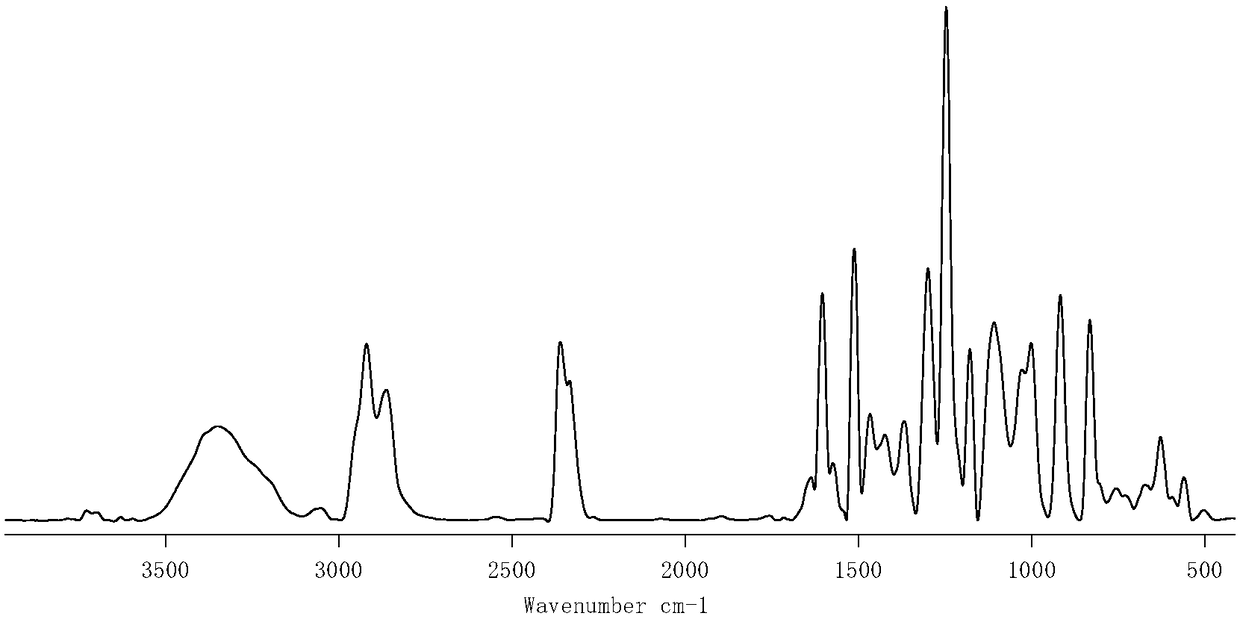
[0102] figure 2 It is the infrared spectrogram of the trifunctional oxim...
Embodiment 3
[0103] The preparation of embodiment 3 thermoplastic oxime-based polyurethanes
[0104] The bifunctional oxime (21 g) prepared in Example 1 was dissolved in 80 mL of tetrahydrofuran solution, hexamethylene diisocyanate (62 g) was added, stirred for 6 h, and the solvent was removed in vacuo to prepare thermoplastic oxime-based polyurethane.
[0105] The chemical reaction equation of the process is shown as follows:
[0106]
[0107] M of the thermoplastic oxime-based polyurethane prepared in this embodiment n = 60,000, PDI = 1.8.
[0108] The structural confirmation data are as follows:
[0109] 1H NMR (400MHz, CDCl 3 ,ppm)δ7.60(d,J=8.4Hz,4H),6.90(d,J=8.4Hz,4H),6.48(s,2H),4.07(t,J=5.6Hz,4H),3.65- 3.60(8H),3.40-3.20(4H),2.74-2.70(8H),2.36(s,6H),2.05(t,J=6.4Hz,4H),1.65-1.55(1H),1.40-1.30(4H ).
[0110] The mechanical properties were tested and the results are shown in Table 1.
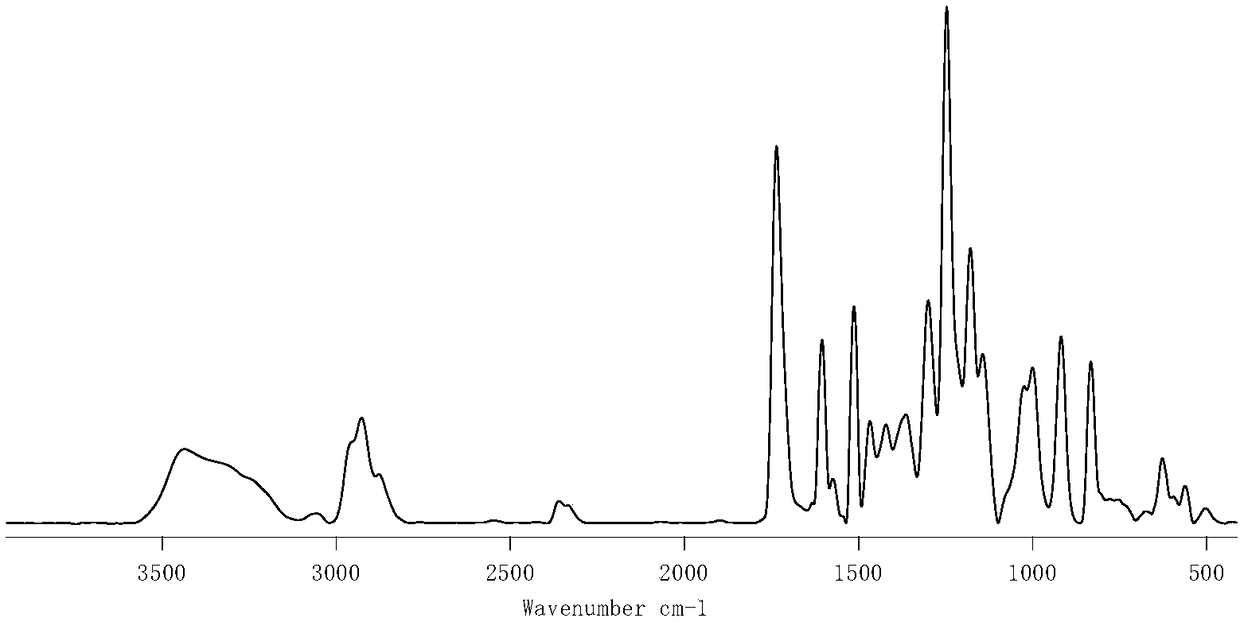
[0111] image 3 It is the infrared spectrogram of the thermoplastic oxime-based polyuretha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mechanical strength | aaaaa | aaaaa |
| Mechanical strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com