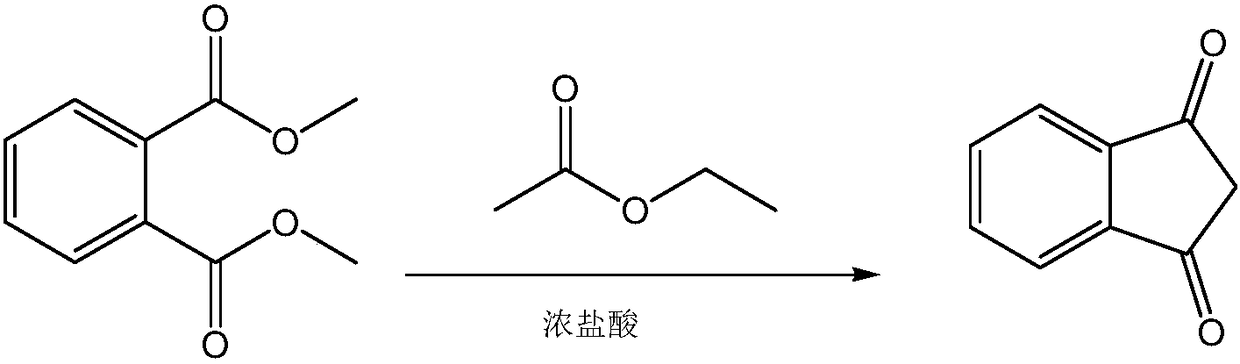
Synthetic process of 1, 3-indandione
A synthesis process, the technology of indenedione, which is applied in the field of synthesis process of 1,3-indenedione, can solve the problems of wasting raw materials and energy consumption, difficult processing, low yield, etc., and achieve resource saving and energy consumption, reaction The effect of condition optimization and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthetic technique of 1,3-indandione comprises the following steps:
[0022] (1) The first step, condensation
[0023] Take 1.5kg of dimethyl phthalate and 1.5L of ethyl acetate, put them into a 10L reaction flask, start stirring, and the inner temperature is 12°C. Then add 918g of sodium methoxide, white turbid liquid, slightly exothermic. Turn on the heating, and stop heating when the temperature rises to 40-50°C. The reaction is exothermic, and the internal temperature rises automatically. When the internal temperature automatically rose to 76°C, the system was a reddish-brown solution, relatively viscous, with bubbles. When the internal temperature automatically rose to 86°C, a large amount of solids precipitated, and the system was yellow and solidified. It was necessary to add ethyl acetate before stirring. A total of 4L of ethyl acetate was added, and the system was a yellow turbid liquid, which was relatively viscous. Keep it warm. React at 70-80°C for ...
Embodiment 2
[0027] Same as Example 1, only modify the mass ratio of dimethyl phthalate, ethyl acetate, and sodium methoxide added to the reaction bottle for the first time in the first condensation reaction to prepare 1,3-indanedione, and calculate its yield ;
[0028] group
Embodiment 3
[0030] Same as Example 1, only modify the mass ratio of ethyl acetate to raw material dimethyl phthalate in the first condensation reaction to prepare 1,3-indanedione, and calculate its yield;
[0031] group
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com