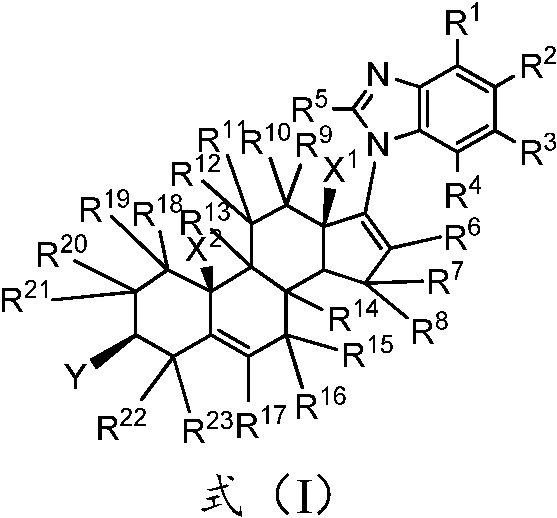
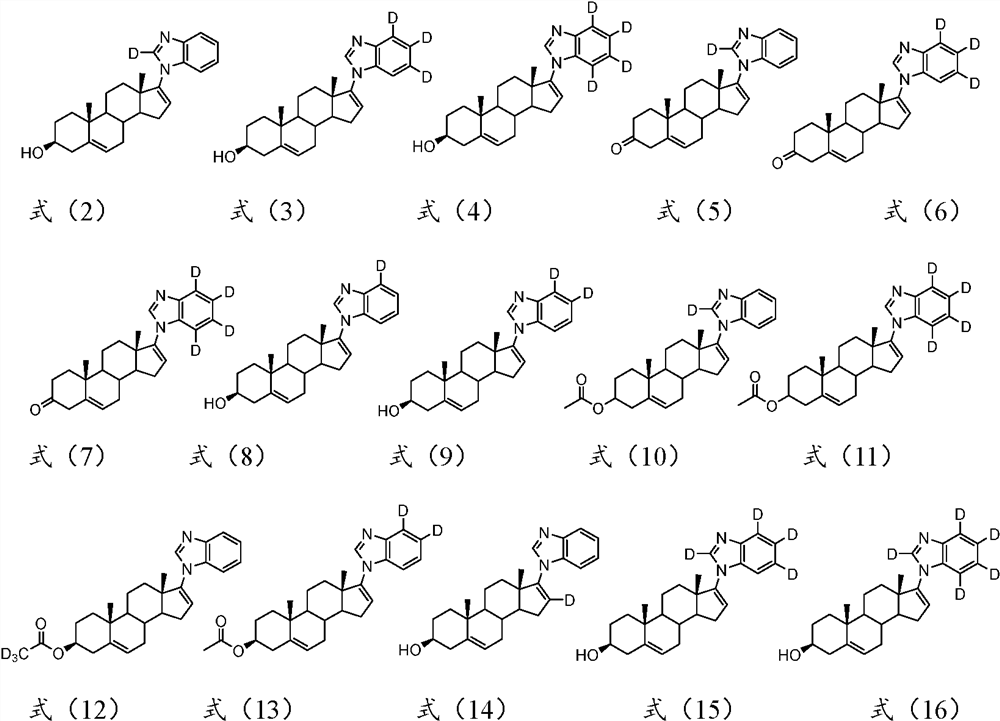
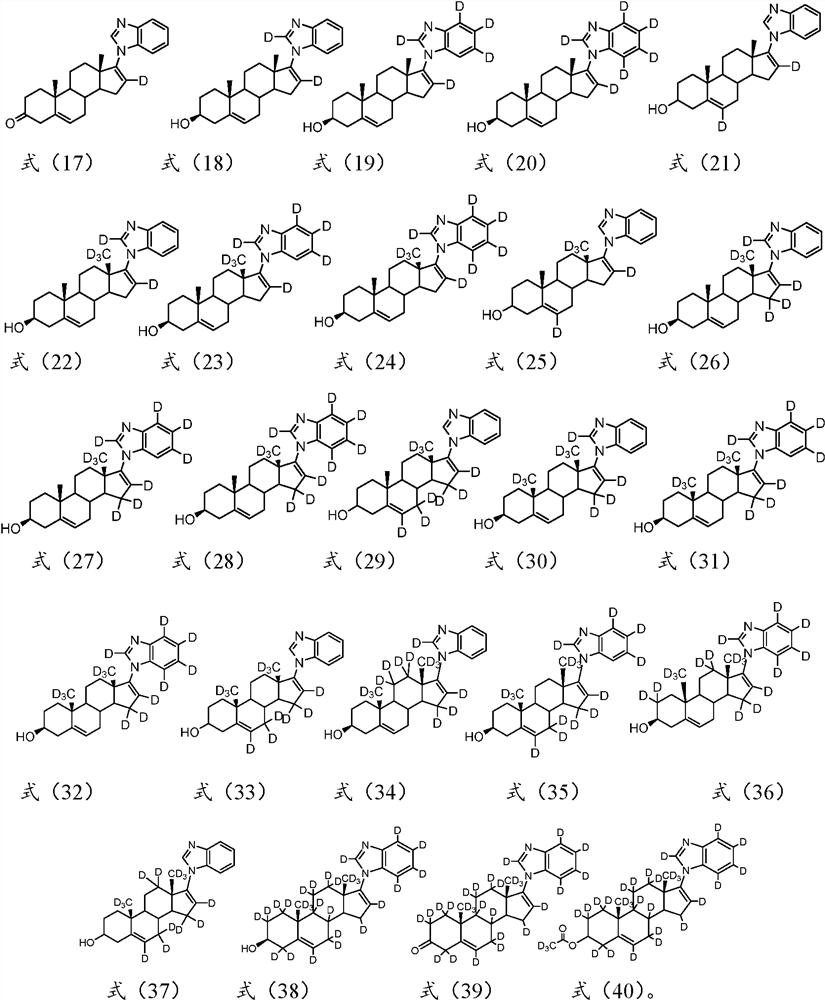
A kind of substituted steroid compound and its application
A compound and composition technology, applied in the field of medicine, can solve the problems of stimulating tumor growth, unable to inhibit androgen, etc., and achieve the effects of improving drug concentration, improving the characteristics of pharmacokinetic parameters, and improving drug efficacy and safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of 3β-17-(1H-benzo[d]imidazol-2-d-1-yl)androst-5,16-dien-3-ol (compound 7)
[0061]
[0062] Step 1: Synthesis of (3β)-3-(acetoxy)-17-chloroandrost-5,16-diene-16-carbaldehyde (compound 2).
[0063] N,N-Dimethylformamide (15 mL, 192 mmol) was slowly added dropwise to a solution of phosphorus oxychloride (15 mL, 165 mmol) in chloroform (45 mL) under ice-cooling and nitrogen protection. After the dropwise addition, a solution of dehydroepiandrosterone acetate (3.00 g, 9.00 mmol) in chloroform (45 mL) was slowly added dropwise. After the dropwise addition was completed, the temperature was raised to room temperature, and the reaction was refluxed for 5 hrs. The reaction solution was concentrated under reduced pressure, the residue was added to ice water, extracted with ether / ethyl acetate (8 / 2, v / v) mixed solvent, the organic layers were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. The organic layer was concent...
Embodiment 2
[0072] Example 2 Preparation of 3β-17-(1H-benzo[d]imidazole-5,6,7-d 3 -1-yl)androst-5,16-dien-3-ol (compound 13)
[0073]
[0074] At room temperature, under nitrogen protection, anhydrous dimethyl sulfoxide (6 mL) was added to 17-iodoandrost-5,16-dien-3β-ol (300 mg, 0.75 mmol), 1H-benzo[d]imidazole- 4, 5, 6, 7-d 4 (110mg, 0.90mmol), L-proline (35mg, 0.3mmol), cuprous iodide (30mg, 0.15mmol) and potassium carbonate (259mg, 1.87mmol) mixture, the reaction solution was overnight at 120°C. Cool to room temperature, add water (25mL) to quench the reaction, filter through celite, extract the filtrate with ethyl acetate (30mLx3), combine the organic layers, wash the organic layer with saturated brine (30mL), dry over anhydrous sodium sulfate, reduce The organic layer was concentrated under pressure, and purified by column chromatography of the concentrate to obtain 80 mg of white solid, yield: 27.2%, purity: 99.37%. LC-MS(APCI): m / z=393.0[M+1] + ; 1 H NMR (300MHz, MeOD-d...
Embodiment 3
[0075] Example 3 Preparation of 3β-17-(1H-benzo[d]imidazole-4,5,6,7-d 4 -1-yl)androst-5,16-dien-3-ol (compound 15)
[0076]
[0077] At room temperature, under nitrogen protection, anhydrous dimethyl sulfoxide (6 mL) was added to 17-iodoandrost-5,16-dien-3β-ol (300 mg, 0.75 mmol), 1H-benzo[d]imidazole- 4, 5, 6, 7-d 4 (110mg, 0.90mmol), L-proline (35mg, 0.3mmol), cuprous iodide (30mg, 0.15mmol) and potassium carbonate (259mg, 1.87mmol) mixture, the reaction solution was overnight at 120°C. Cool to room temperature, add water (25mL) to quench the reaction, filter through celite, extract the filtrate with ethyl acetate (30mLx3), combine the organic layers, wash the organic layer with saturated brine (30mL), dry over anhydrous sodium sulfate, reduce The organic layer was concentrated under pressure, and purified by column chromatography of the concentrate to obtain 80 mg of white solid, yield: 27.2%, purity: 99.37%. LC-MS(APCI): m / z=393.0[M+1] + ; 1 H NMR (300MHz, MeOD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com