Artificial fusion protein and application thereof
A fusion protein and artificial technology, applied in the field of biomedicine, can solve the problems of reduced biological activity, low reaction yield, difficult administration of interferon-α2, etc., and achieve the effect of increasing half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Physicochemical Characterization of IFNα-Sup35
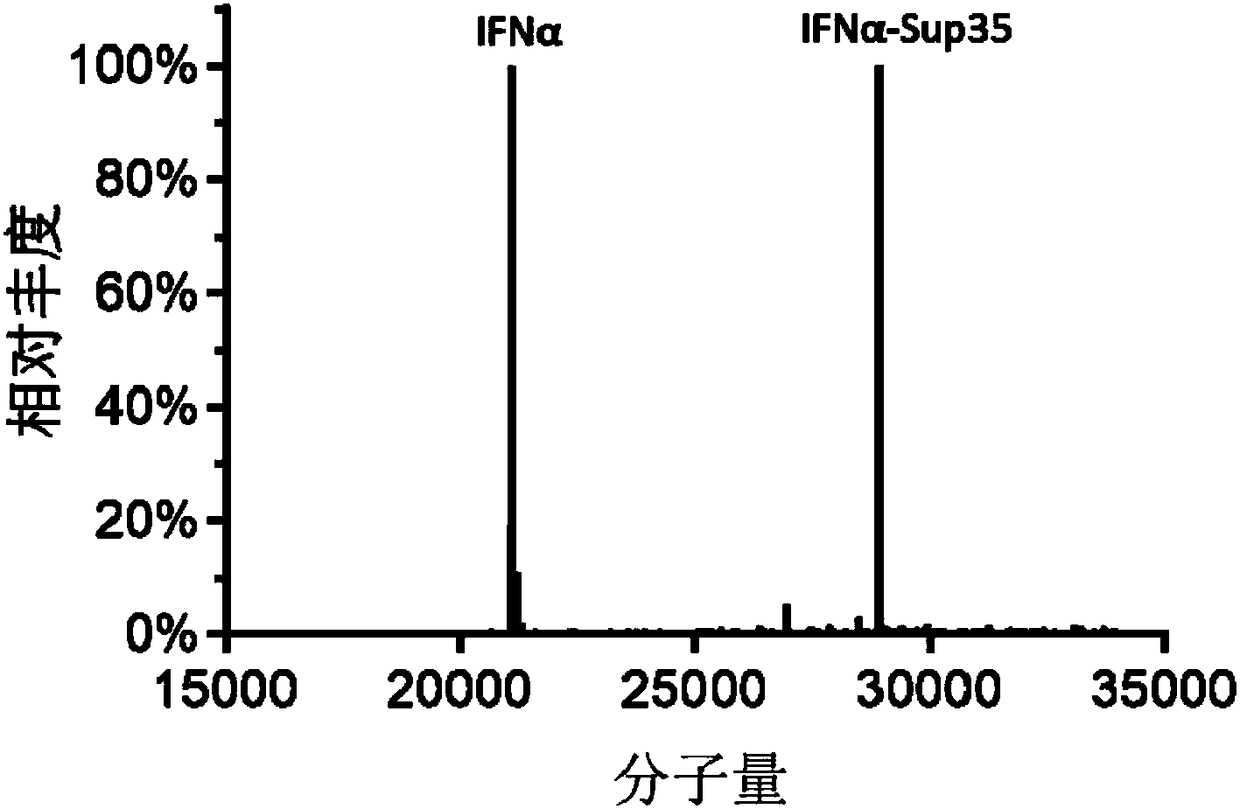
[0098] The molecular weight of IFNα and IFNα-Sup35 was determined by quadrupole / ionization time-of-flight mass spectrometry (Q-TOF). The samples were first separated by nanoACQUITY high-performance liquid chromatography system, and then separated by SYNAPT-G2-Si mass spectrometer. The mobile phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 100% acetonitrile and 0.1% formic acid. Afterwards, the samples were placed in an autosampler for electrospray ionization and analyzed in a Q-TOF (SYNAPT G2-Si, Waters company) mass spectrometer. image 3 The Q-TOF analysis results are shown.
[0099] The hydration radius of IFNα and IFNα-Sup35 was determined by dynamic light scattering (DLS) method on Malvern Zetasizer Nano-zs90. Samples were diluted in PBS buffer and filtered through a 0.22 μm pore size filter before testing. As tested by DLS, the hydrated diameter of IFNα is 7nm, while the ...
Embodiment 2
[0103] Example 2 In vitro biological activity measurement and biological safety of IFNα-Sup35
[0104] The anti-cell proliferation activity and biological safety of IFNα-Sup35 in the present invention are determined by MTT method. We chose human Burkitt's B lymphoma cells (Daudi B) to test the anti-proliferation activity of IFNα-Sup35NP, because the cells are highly sensitive to IFN-α2. Human mammary epithelial cells (L929) and mouse fibroblasts (MCF-10) Daudi B cells were used to test the biological safety of IFNα-Sup35NP. After the cells were cultured in RMPI-1640 containing 10% FBS, 50 U / mL penicillin and 50 μg / mL streptomycin for a period of time (L929 and MCS-10 were cultured in DMEM medium), a certain concentration of The cell suspension (50 μL / well, 104 cells), the IFNα and IFNα-Sup35NP samples were serially diluted, 50 μL each was added to a 96-well culture plate, and a negative control (without IFN-α2) and a blank control (only cultured solution), 37°C, 5% CO2 for 7...
Embodiment 3
[0107] Example 3 Pharmacokinetic test of IFNα-Sup35NP
[0108] All the following animal experiments were completed under the guidance of Tsinghua University's regulations on animal experiments. In the present invention, the BABL / C athymic female nude mouse model was used to inject IFNα or IFNα-Sup35NP through the tail vein, and the change of interferon concentration in blood over time was measured, and the data was analyzed by DAS software. Before drug treatment, six 8-week-old male BABL / C with a body weight of about 20 g were observed for a period of time and randomly divided into two groups. IFNα and IFNα-Sup35NP were injected into the tail vein at a dose of 50 μg / 20 g, and then 10 μL of blood was collected by docking the tail at the set time point, left at room temperature for 30 min, and the supernatant serum was collected by centrifugation at 4°C and 3000×g. Store in a -80°C low-temperature refrigerator. The content of IFN-α2 in the serum was measured with the human IFN...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com