Method for synthesizing D(+)beta -(3,4-Dihydroxyphenyl) lactic acid by enzymic method
A technology of enzymatic synthesis and danshensu, applied in microorganism-based methods, biochemical equipment and methods, enzymes, etc., can solve the problems of high efficiency, shortage of raw materials, low cost, etc., to solve the problems of high price and high molar conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 A method for enzymatically synthesizing Danshensu, the specific steps are as follows:
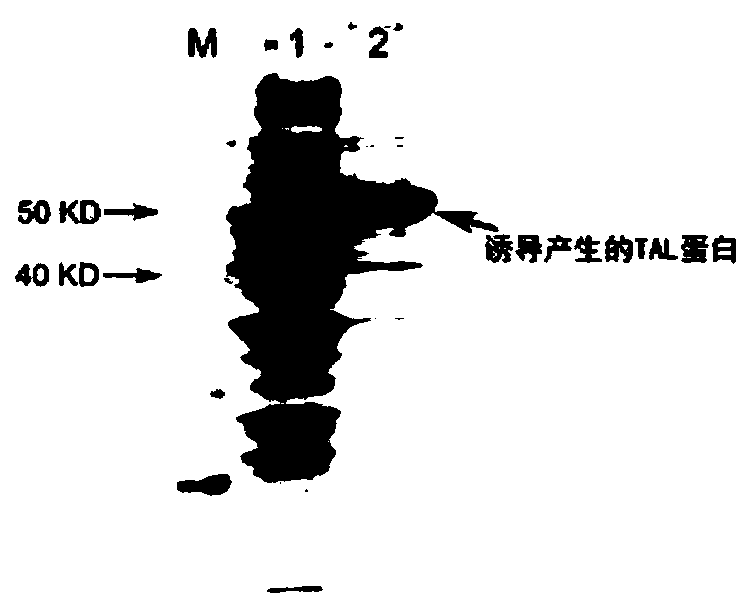
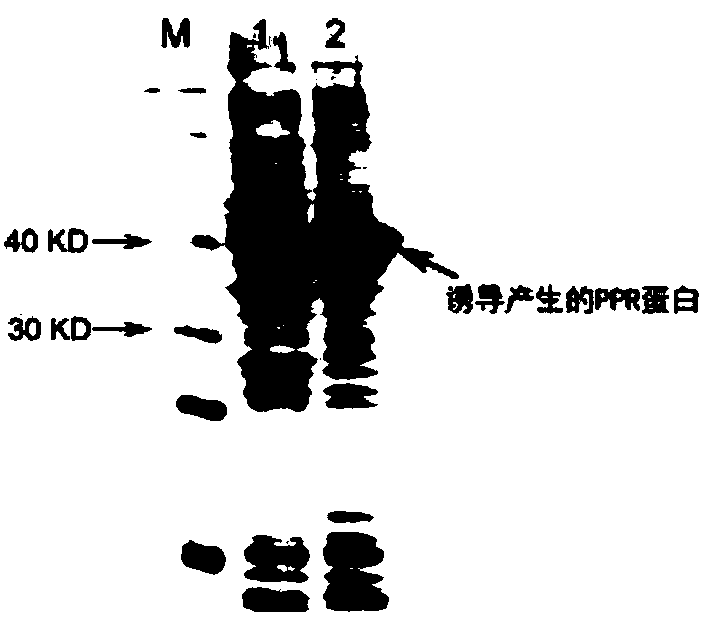
[0049] 1. Heterologous expression of tyrosine ammonia lyase TAL-made, phenylpyruvate reductase PPR-made and glucose dehydrogenase GDH-made:
[0050] 1. Heterologous expression and enzyme activity determination of tyrosine ammonia lyase TAL-made:
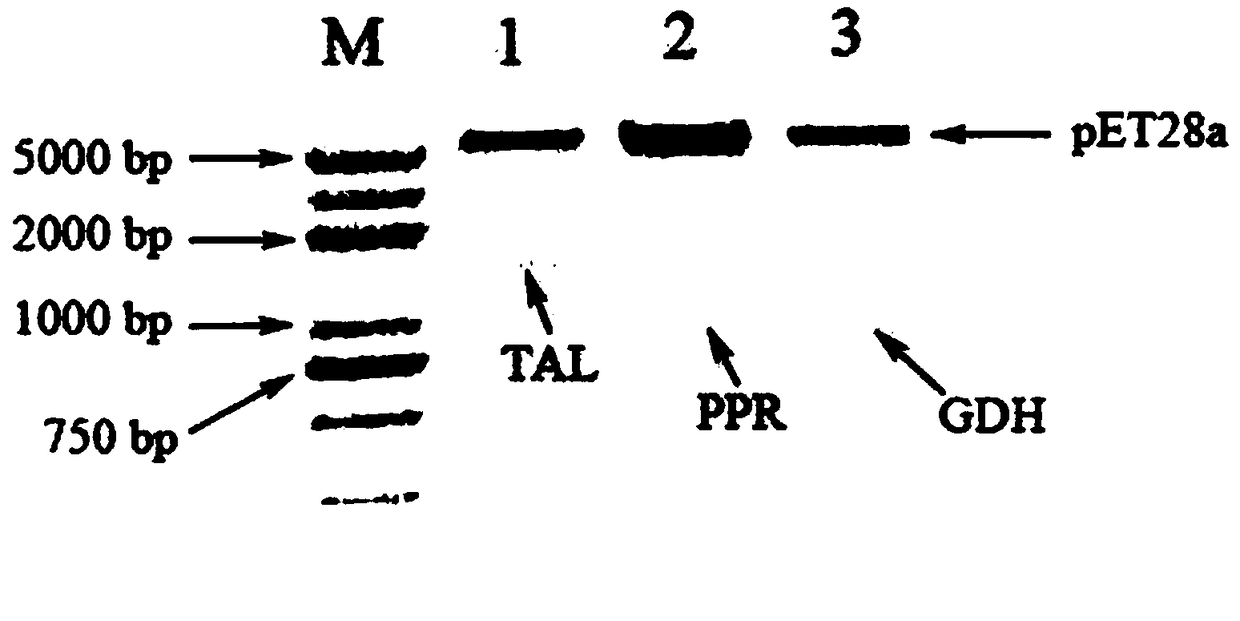
[0051] (1) Obtain TAL-made gene fragment
[0052] According to the tyrosine ammonia lyase (TAL) gene sequence (Genbank accession number: CP015288, SEQ ID NO.1), the TAL-made gene (SEQ ID NO. 2) The amino acid sequence of the gene is completely identical to that of the TAL gene, and BamHI and XhoI restriction enzyme sites are introduced upstream and downstream of the gene sequence to facilitate subsequent vector construction.
[0053] (2) Construction of pET28a-TAL-made expression vector
[0054] The synthesized TAL-made gene and the pET28a vector were digested with BamHI and XhoI respectively, and after ligation and transfor...
Embodiment 2
[0074] Except that the following steps are different, all the other steps are the same as in Example 1.
[0075] Obtain the expression cells of TAL, PPR and GDH according to the method in step 1, and add the following drug preparation catalyst systems in turn to the 250ml Erlenmeyer flask: levodopa 1.0mol, amino acid deaminase wet cells 20g / L, phenylpyruvate Reductase wet cell 20g / L, glucose dehydrogenase wet cell 20g / L, adjust the pH of the reaction system to 8.0, transform at 30°C for 16h, the obtained Danshensu content is 58.0g / L, and the molar conversion rate is greater than 58.1 %.
Embodiment 3
[0077] Except that the following steps are different, all the other steps are the same as in Example 1.
[0078] Obtain the expression cells of TAL, PPR and GDH according to the method in step 1, and add the following medicine preparation catalyst system successively in the 250ml Erlenmeyer flask: Levodopa 1.5mol, amino acid deaminase wet cell 30g / L, phenylpyruvate Reductase wet cell 30g / L, glucose dehydrogenase wet cell 30g / L, adjust the pH of the reaction system to 8.0, transform at 30°C for 16h, the content of Danshensu obtained is 60.7g / L, and the molar conversion rate is greater than 60.8 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com