Compound ointment preparation and preparation method thereof
An ointment and preparation technology, applied in the field of pharmaceutical preparations, can solve problems such as inability to uniformly disperse, agglomeration and hardening of lidocaine hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of described glycidyl methacrylate modified carboxymethyl chitosan comprises the following steps:
[0040] Add carboxymethyl chitosan with a number average molecular weight of 9000 and purified water into the reactor, raise the temperature to 50°C, stir for 0.5h, cool down to 30°C, add triethylamine, glycidyl methacrylate, tetrabutyl Ammonium bromide, heat preservation reaction for 72h, and then ethanol precipitation, deionized water dialysis for 48h, freeze-drying at -42°C for 48h, and freeze-drying to obtain the glycidyl methacrylate modified carboxymethyl chitosan; The weight ratio of carboxymethyl chitosan to the purified water, the triethylamine, the glycidyl methacrylate, the tetrabutylammonium bromide, and the dehydrated alcohol is 1:50:3 :5:5:50.
[0041] The preparation method of described glycidyl ether modified carboxymethyl chitosan comprises the following steps:
[0042] Add carboxymethyl chitosan with a number average molecular wei...
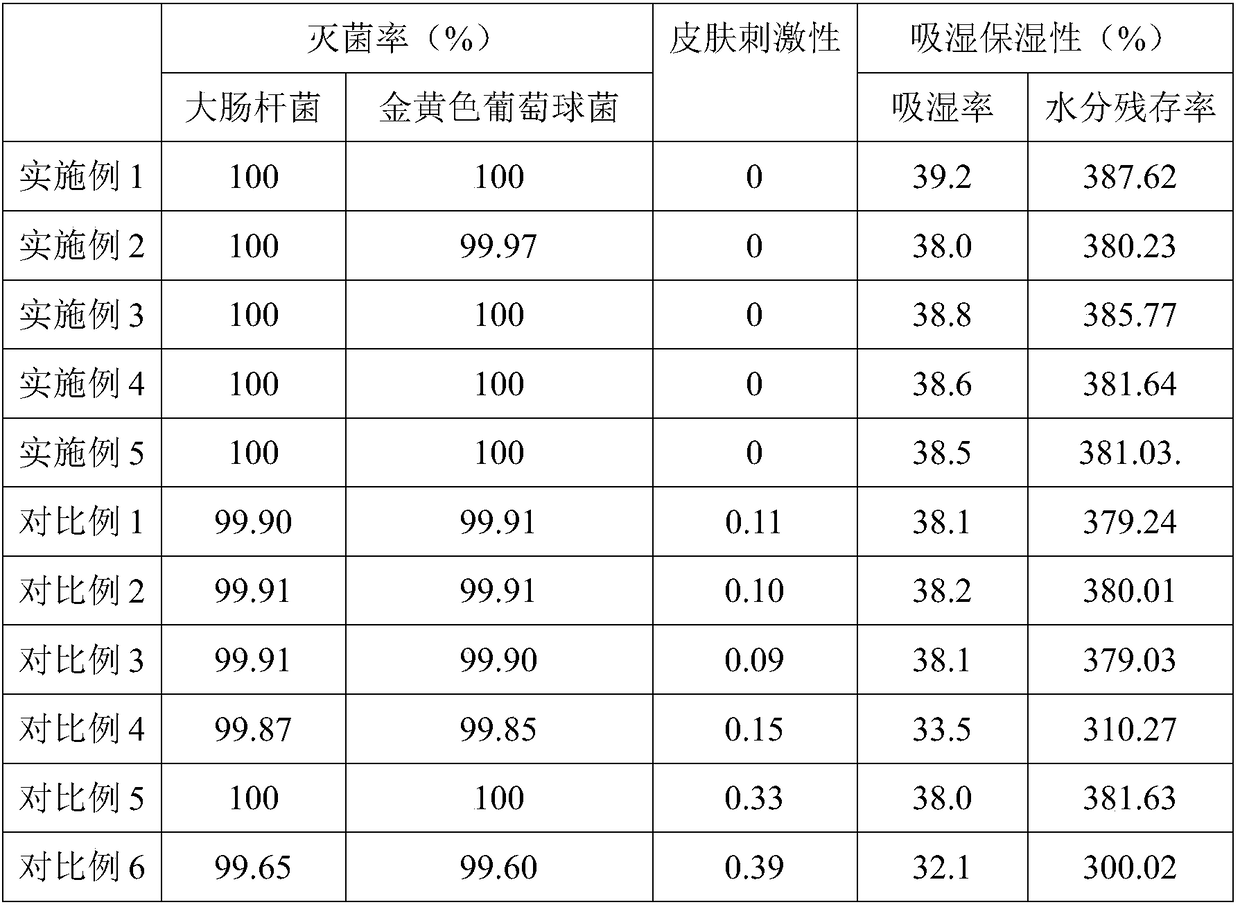
Embodiment 1
[0062] The weight of each component in each kilogram of ointment preparation in the described compound ointment preparation comprises: 5 million units of polymyxin B sulfate, 3.5 million units of neomycin sulfate, 500,000 units of bacitracin, 40 g of lidocaine hydrochloride, matrix added to Prescription amount;
[0063] Wherein, the base is white petrolatum, liquid paraffin, α-cyclodextrin, modified chitosan, propylene glycol, methyl p-hydroxybenzoate; the white petrolatum and the liquid paraffin, the α-cyclodextrin , the weight ratio of the modified chitosan, the propylene glycol, and the methyl p-hydroxybenzoate is 1:0.3:0.024:0.16:0.07:0.003; the modified chitosan includes glycidyl methacrylate Ester modified carboxymethyl chitosan, glycidyl ether modified carboxymethyl chitosan, arginine and gluconic acid modified carboxymethyl chitosan; the glycidyl methacrylate modified carboxymethyl chitosan The weight ratio of chitosan to the glycidyl ether modified carboxymethyl chit...
Embodiment 2
[0076] The weight of each component in each kilogram of ointment preparation in the described compound ointment preparation comprises: 5 million units of polymyxin B sulfate, 3.5 million units of neomycin sulfate, 500,000 units of bacitracin, 40 g of lidocaine hydrochloride, matrix added to Prescription amount;
[0077] Wherein, the base is white petrolatum, liquid paraffin, α-cyclodextrin, modified chitosan, propylene glycol, methyl p-hydroxybenzoate; the white petrolatum and the liquid paraffin, the α-cyclodextrin , the weight ratio of the modified chitosan, the propylene glycol, and the methyl p-hydroxybenzoate is 1:0.3:0.024:0.05:0.07:0.003; the modified chitosan includes glycidyl methacrylate Ester modified carboxymethyl chitosan, glycidyl ether modified carboxymethyl chitosan, arginine and gluconic acid modified carboxymethyl chitosan; the glycidyl methacrylate modified carboxymethyl chitosan The weight ratio of chitosan to the glycidyl ether modified carboxymethyl chit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com