Catalyst for selective hydrogenation reaction of phthalate and preparation method of catalyst
A phthalate ester and hydrogenation reaction technology, applied in the field of catalysis, can solve the problems of high loss rate and poor stability of precious metals in the catalyst, and achieve the effects of excellent stability, good activity and low loss rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
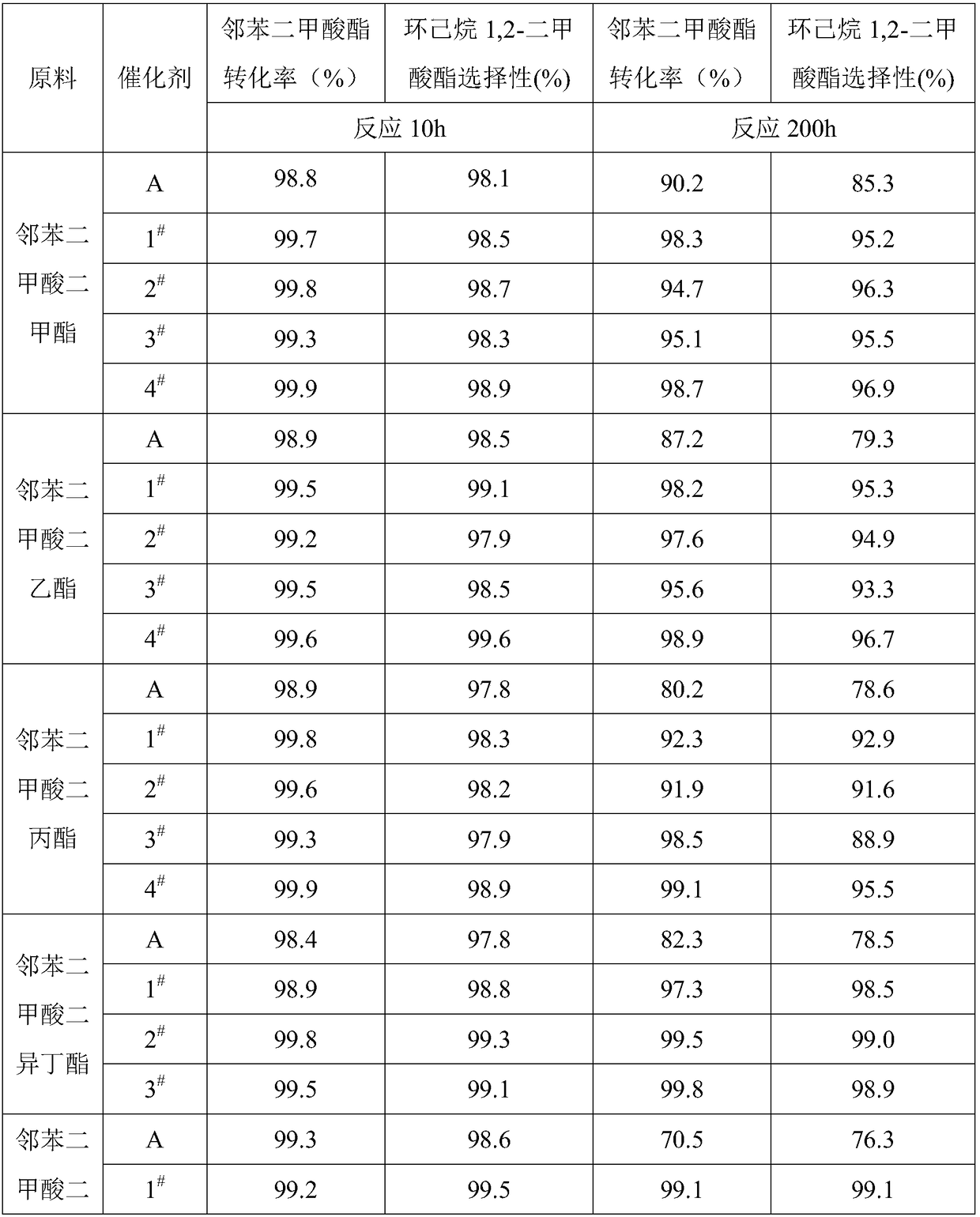
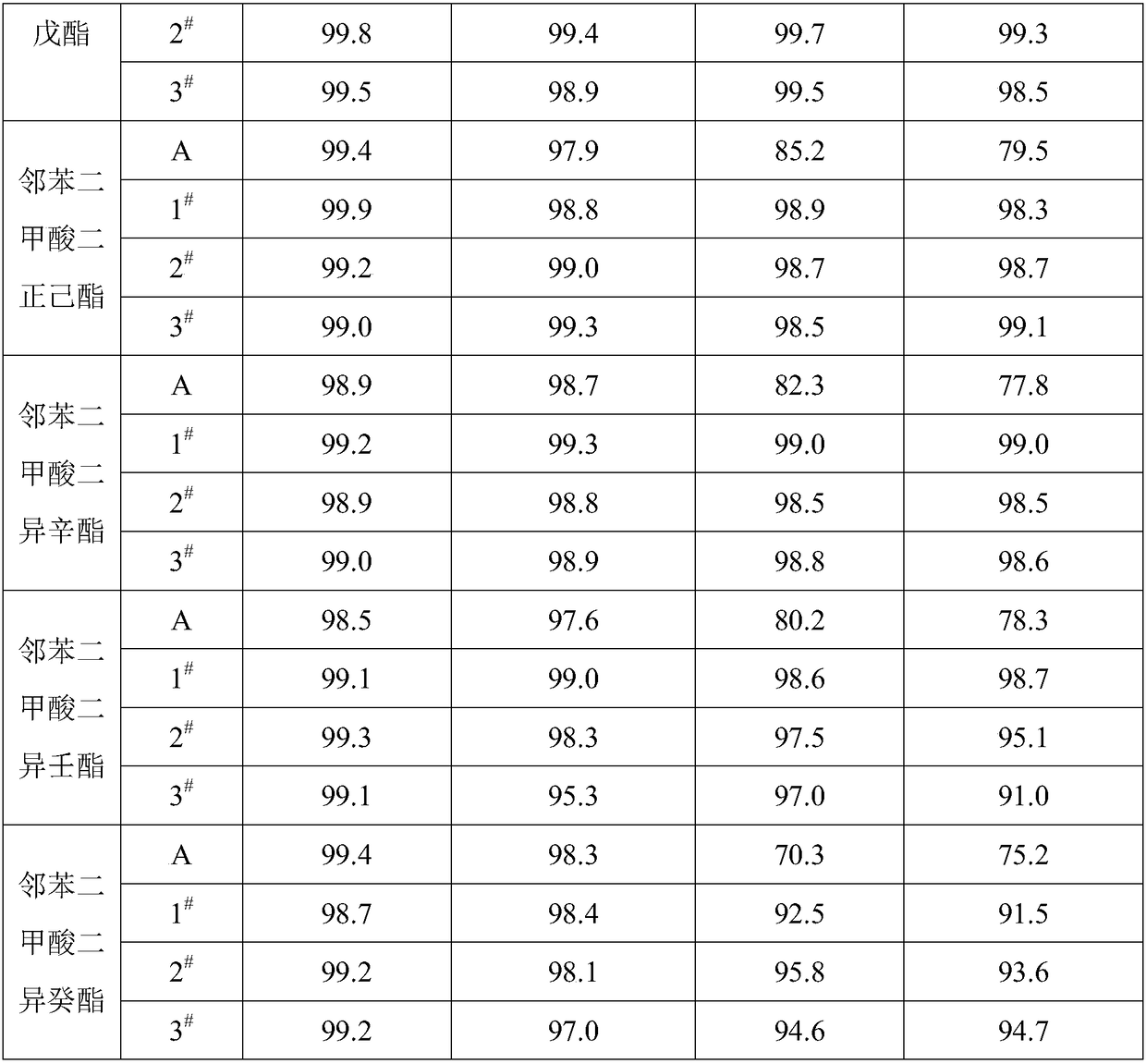
[0027] 1) Take 2g HY molecular sieve and 2g silicalite-1 molecular sieve, heat treatment at 120℃ for 1h; 2) Add 2g γ-alumina, 1g triethylhexyl phosphoric acid and 4g deionized water to the mixed molecular sieve after heat treatment , kneaded evenly; 3) Granulate the kneaded material into particles with a particle size of about 1.2 mm, and dry at 120 ° C for 3 hours; 4) Add 12 g of graphite to the dried particles and mix them evenly, and press them into Ф5× 5mm cylindrical particles; 5) The tablet is roasted at 700°C to obtain the carrier required for the preparation of the catalyst; 6) The carrier is immersed in a mixed solution containing ruthenium chloride, rubidium chloride, phosphoric acid, sodium chloride, and lanthanum nitrate for 0.5h , taken out and dried at 120°C, and then roasted at 200°C for 2h; the mass content of ruthenium element is 0.1%, the mass content of rubidium element is 0.1%, the mass content of phosphorus element is 0.1%, and the mass content of sodium el...
Embodiment 2
[0029] 1) Take 10g HY molecular sieve and 10g silicalite-1 molecular sieve, heat treatment at 700°C for 3h; 2) Add 10g aluminum stearate, 2g sodium lauryl sulfate and 30g sodium lauryl sulfate to the mixed molecular sieve after heat treatment Ionized water, kneaded evenly; 3) Granulate the uniformly kneaded material into particles with a particle diameter of about 1.2 mm, and dry at 120 ° C for 5 hours; 4) Add 12 g of graphite to the dried particles and mix them evenly, and press them into tablets by a tablet machine. Ф5×5mm cylindrical particles; 5) The tablet is calcined at 1200°C to obtain the carrier required for the preparation of the catalyst; 6) The carrier is immersed in the mixed solution of chloroplatinic acid, strontium chloride, magnesium chloride, cerium nitrate, and boric acid for 3 hours, and taken out Dry at 120°C, and then bake at 600°C for 4 hours; the mass content of platinum element is 5%, the mass content of strontium element is 2%, the mass content of boro...
Embodiment 3
[0031]1) Take 5g HY molecular sieve and 5g silicalite-1 molecular sieve, heat treatment at 500°C for 2h; 2) add 5g aluminum nitrate, 1.5g methyl amyl alcohol and 15g deionized water to the mixed molecular sieve after heat treatment, knead 3) Granulate the uniformly kneaded material into particles with a particle size of about 1.2mm, and dry at 120°C for 4 hours; 4) Add 12g of graphite to the dried particles and mix them evenly, and press them into Ф5×5mm particles by a tablet press Cylinder granules; 5) the tablet is roasted at 1100°C to obtain the carrier required for the preparation of the catalyst; 6) the carrier is immersed in the mixed solution of palladium chloride, potassium chloride, yttrium chloride, selenium nitrate and cerium nitrate for 2 hours, and taken out drying at 120°C, and then roasting at 550°C for 3 hours; the mass content of palladium element is 2.5%, the mass content of yttrium element is 1%, the mass content of selenium element is 1%, and the mass conten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com