Method for determining aminoglycoside drug in animal muscle tissue without using of ion pair reagent
A technology of aminoglycosides and ion-pair reagents, which is applied in the field of determination of aminoglycosides in animal muscle tissue without using ion-pairing reagents, and can solve problems such as difficult reversed-phase column retention, human hazards, and residues of aminoglycosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
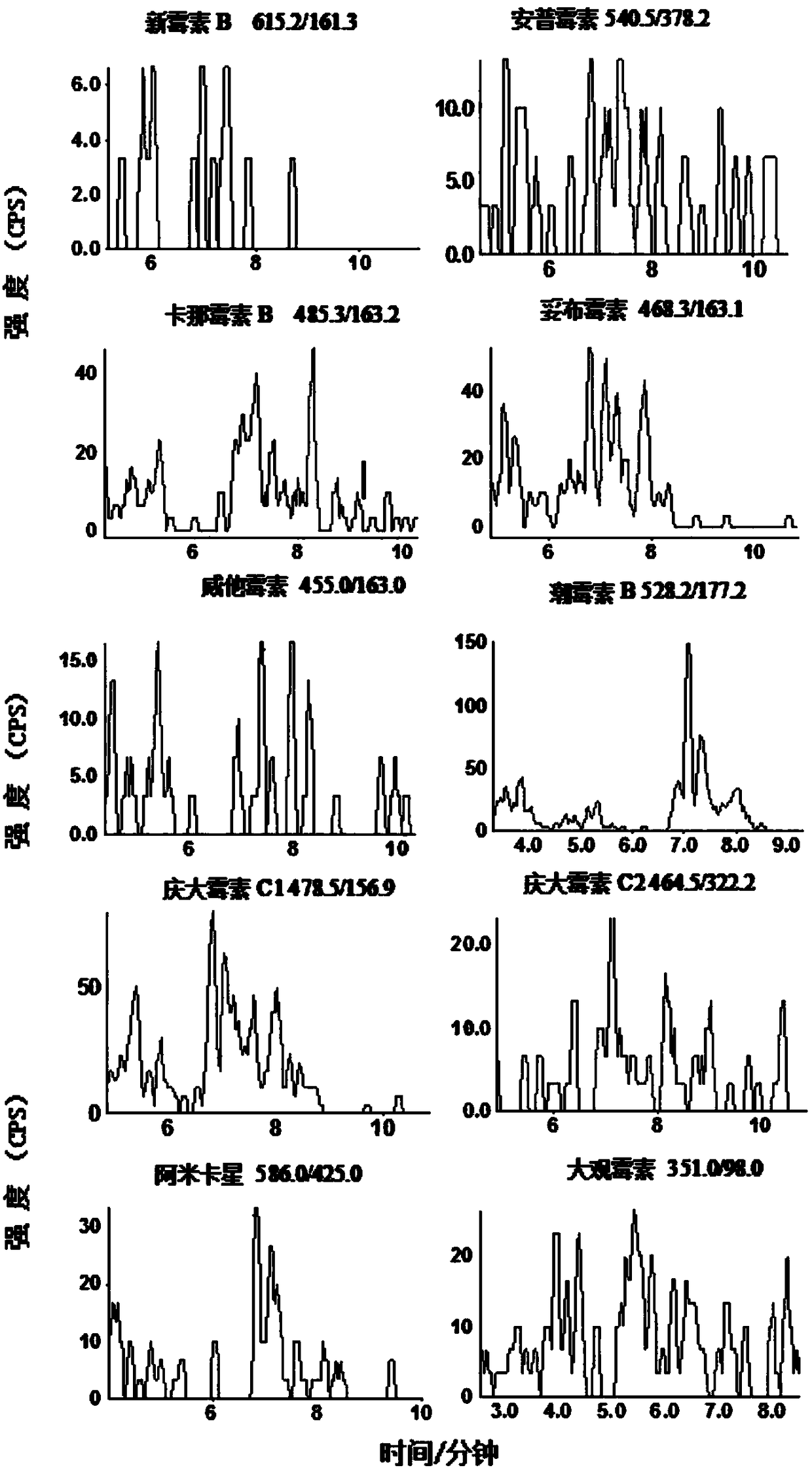
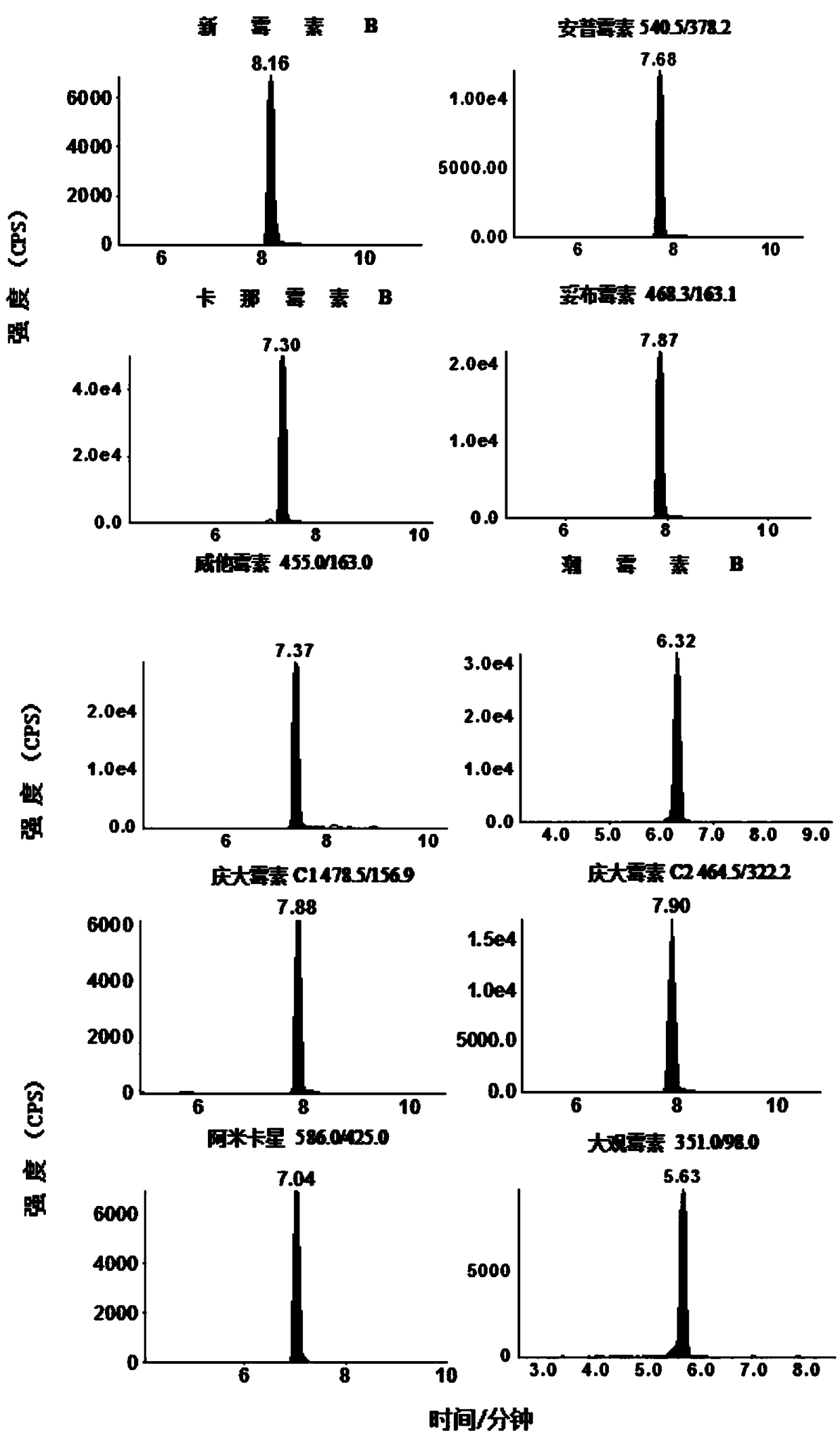
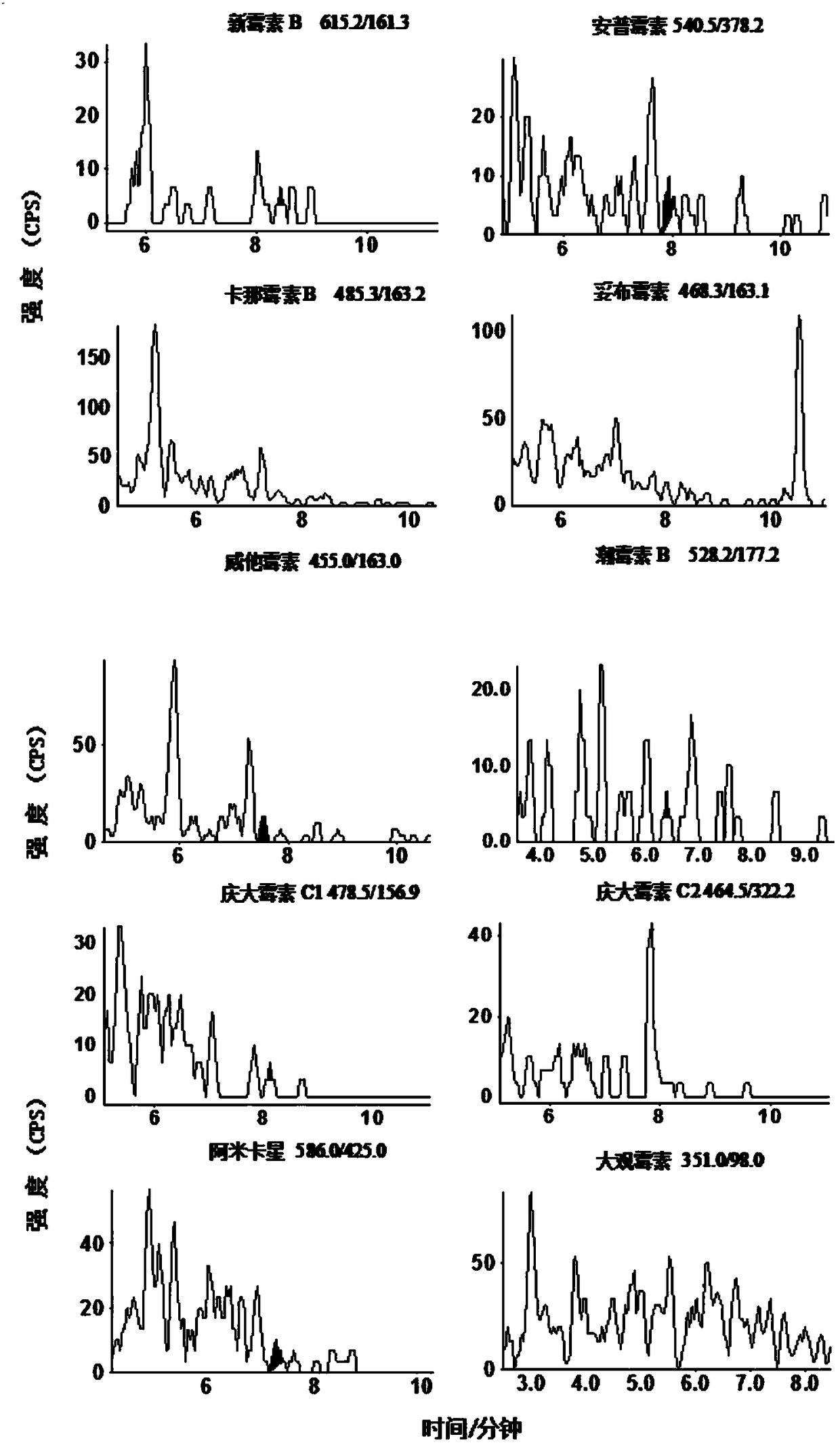
Image
Examples
preparation example Construction
[0072] In the present invention, the preparation method of the described sample injection includes:
[0073] 1) Mix the animal muscle tissue with the phosphate extract, perform the first ultrasonic treatment for 8-12 minutes to obtain the first ultrasonic treatment product, and centrifuge the first ultrasonic treatment product to obtain the first precipitate and the first clear liquid ;
[0074] 2) Mix the first precipitate obtained in step 1) with the phosphate extract, perform the second ultrasonic treatment for 8 to 12 minutes to obtain the second ultrasonic treatment product, and centrifuge the second ultrasonic treatment product to obtain the second ultrasonic treatment product. Second precipitation and second clear solution;
[0075] 3) mixing the first clear liquid obtained in step 1) with the second clear liquid obtained in step 2) to obtain a mixed clear liquid;
[0076] 4) Add the mixed clear liquid obtained in step 3) into the MCX column, rinse with acetic acid so...
Embodiment 1
[0105] The percentage concentration of the solution involved in this embodiment is volume percentage concentration unless otherwise specified.
[0106] 1. Preparation of standard solution
[0107] Precisely weigh 10 mg each of neomycin B, apramycin, kanamycin B, tobramycin, vertamycin, hygromycin B, gentamicin, amikacin, and spectinomycin, Dilute the volume in a 10mL brown volumetric flask with ultrapure water and mix well to obtain a standard stock solution with a concentration of 1mg / mL for each drug, and dilute it with ultrapure water to form a mixed standard working solution with an appropriate mass concentration, -20°C Store away from light, valid for 1 month.
[0108] 2. Preparation of phosphate extract
[0109] Accurately weigh 1.36 g of potassium dihydrogen phosphate, dissolve it in 980 mL of water, add 0.15 g of disodium edetate and 20 g of trichloroacetic acid in sequence, fully dissolve and mix, and dilute to 1 L with ultrapure water.
[0110] 3. Sample Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com