Preparation method of econazole nitrate
A technology of econazole nitrate and dilute nitric acid, applied in directions such as organic chemistry, can solve the problems of low yield, high viscosity and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
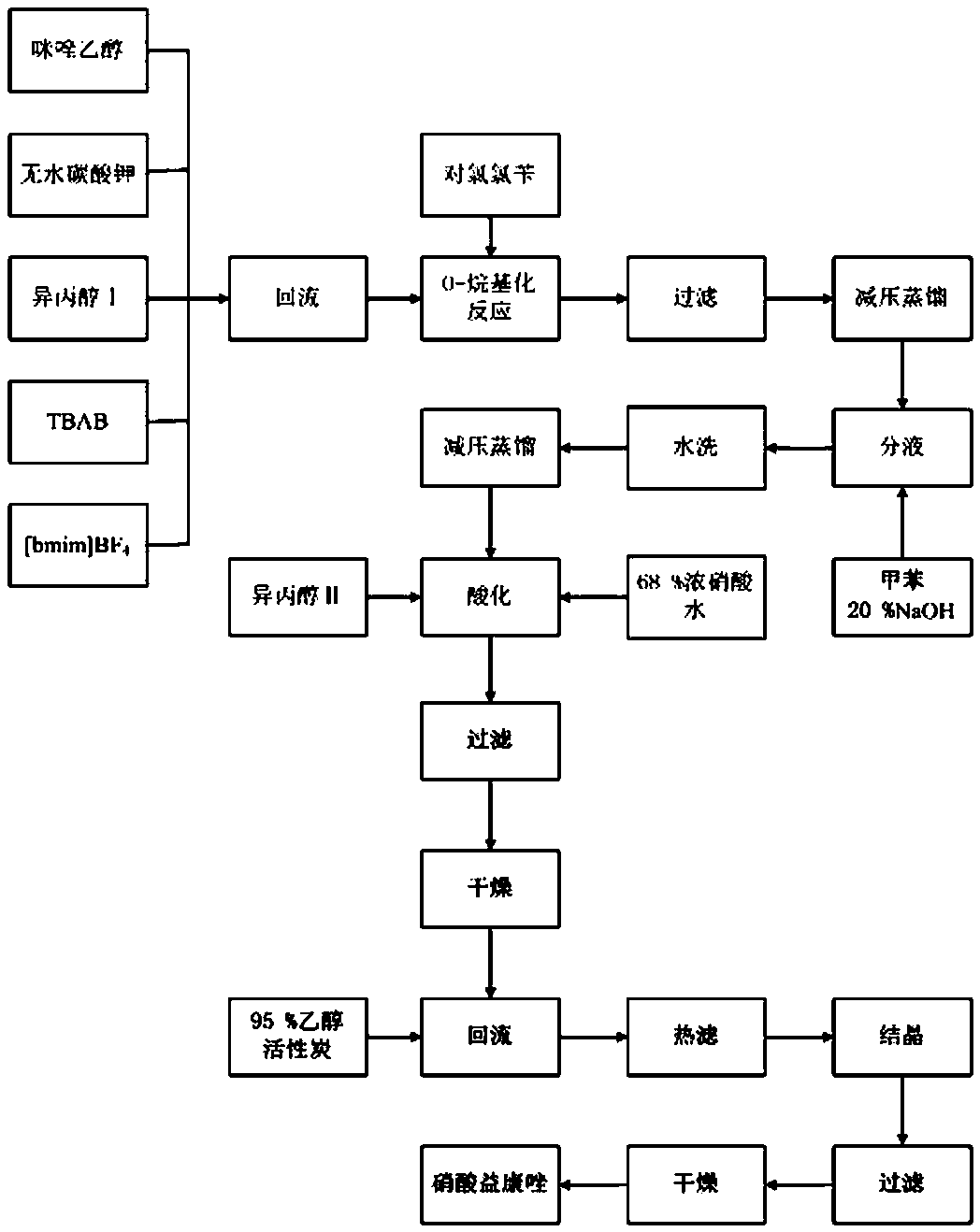
[0025] Add 51.4 g imidazole ethanol, 35 g anhydrous potassium carbonate, 105 g isopropanol Ⅰ, 1.5 g TBAB, 3.5 g [bmim] BF into a 500 mL four-necked reaction flask with mechanical stirring and reflux condenser 4 , heated to reflux for 1 h, cooled to 75 ° C, added 34 g p-chlorobenzyl chloride, reacted at 72 ± 1 ° C for 4.0 h, filtered, recovered isopropanol by distillation under reduced pressure, added 160 g toluene, 20% NaOH solution, stirred, Wash with water until neutral, distill the supernatant under reduced pressure to obtain free alkali oil, add 140 g of isopropanol II to dissolve, use 18 g of concentrated nitric acid with a mass fraction of 68% and 23 g of water to prepare dilute nitric acid with a mass fraction of 30% for acidification , until the precipitation stopped, filtered, washed with water, and dried to obtain the crude product of econazole nitrate.
[0026] Add the obtained econazole nitrate crude product and 230 g 95% ethanol in a 500 mL four-necked reaction fl...
Embodiment 2
[0028] Add 51.4 g imidazole ethanol, 30 g anhydrous potassium carbonate, 115 g isopropanol Ⅰ, 2.5 g TBAB, 2.0 g [bmim] BF 4 , heated to reflux for 1 h, cooled to 75 ° C, added 34.5 g p-chlorobenzyl chloride, reacted at 73 ± 1 ° C for 4.5 h, filtered, and recovered isopropanol by distillation under reduced pressure, added 170 g toluene, 20 % NaOH solution, stirred, Wash with water until neutral, distill the supernatant under reduced pressure to obtain free alkali oil, add 130 g of isopropanol II to dissolve, and use 22 g of concentrated nitric acid with a mass fraction of 68% and 28 g of water to prepare dilute nitric acid with a mass fraction of 30% for acidification , until the precipitation stopped, filtered, washed with water, and dried to obtain the crude product of econazole nitrate.
[0029] Add the obtained econazole nitrate crude product and 206 g95% ethanol in the 250 mL four-necked reaction flask with mechanical stirring and reflux condenser, stir, and be heated to t...
Embodiment 3
[0031] Add 51.4 g imidazole ethanol, 40 g anhydrous potassium carbonate, 125 g isopropanol I, 2.0 g TBAB, 2.5 g [bmim] BF into a 250 mL four-necked reaction flask with mechanical stirring and reflux condenser 4 , heated to reflux for 1 h, cooled to 75 °C, added 35 g of p-chlorobenzyl chloride, reacted at 74±1 °C for 4.0 h, filtered, recovered isopropanol by vacuum distillation, added 175 g of toluene, 20 % NaOH solution, stirred, washed with water To neutrality, the supernatant liquid is distilled under reduced pressure to obtain free alkali oil, add 135 g isopropanol II for dissolving, and use 20.5 g mass fraction as 68% concentrated nitric acid and 26 g water to be mixed with 30% dilute nitric acid for acidification, After the precipitation stopped, filter, wash with water, and dry to obtain the crude product of econazole nitrate.
[0032] Add the obtained econazole nitrate crude product and 220 g95% ethanol in the 250 mL four-necked reaction flask with mechanical stirring a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com