Pseudoionone synthesis method
A technique for the synthesis of pseudoionones and synthetic methods, which is applied in the field of synthesis of pseudoionones, can solve the problems of long time, large amount of acetone, and poor atom economy, and achieve high production efficiency, fewer reaction steps, and improved purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
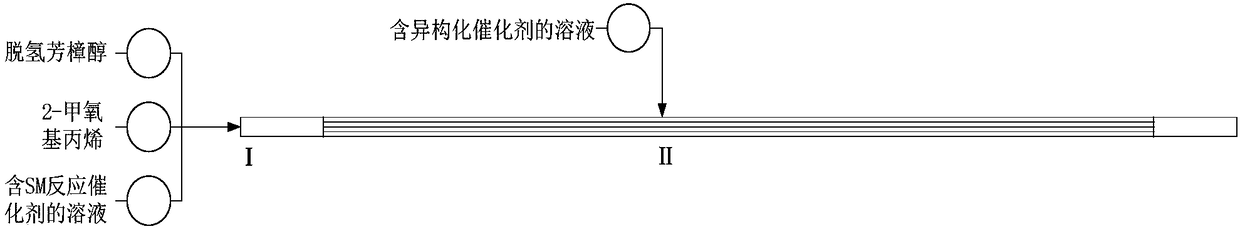
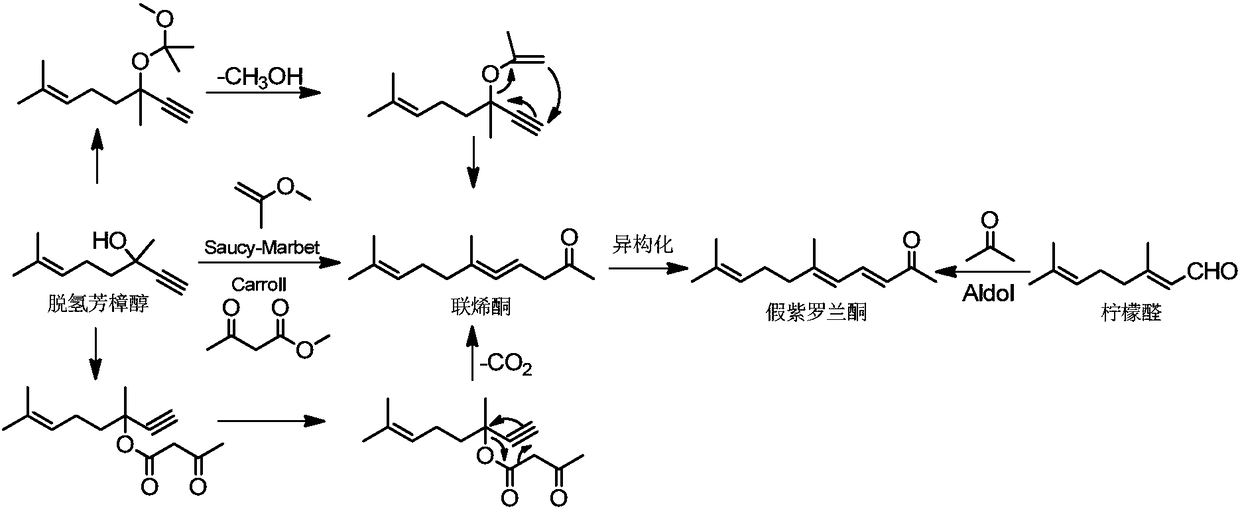
[0029] Dehydrolinalool, 2-methoxypropene and 2,2-dimethoxypropane solution of methanesulfonic acid with a mass content of 0.133% (molar ratio is 1:2.3:0.001) were prepared at 0.057ml / min, The flow rates of 0.070ml / min and 0.049ml / min are pumped into the microchannel reactor at 160°C (the pipe length is 28m*5, the inner diameter is 0.2*0.2mm, the pressure is about 0.8MPa, and the feed port (II) is set at 1 / 2 locations). The sample was taken from the sampling port of the pipeline, and through gas analysis, it was confirmed that the products were allenone and pseudopurple isomers. The collected solution was analyzed by gas phase, and the conversion rate of dehydrolinalool was 99.5%, and the selectivity for the products diketenone and pseudopurple was 96.5%.
[0030] Pass into the 2,2-dimethoxypropane solution (the mol ratio of dehydrolinalool and phosphoric acid is 1:0.002) of the phosphoric acid of 0.242% from the feed port (II), flow is 0.039ml / min. The collected solution ...
Embodiment 2
[0032] Dehydrolinalool, 2-methoxypropene and 2,2-dimethoxypropane solution of phosphoric acid with a mass fraction of 0.242% (molar ratio is 1:2.3:0.002), respectively at 0.057ml / min, 0.070 The flow rate of ml / min and 0.049ml / min is pumped into the microchannel reactor at 160°C (the tube length of the microchannel reactor is 28m*5, the inner diameter of the tube is 0.2*0.2mm, and the pressure inside the tube is maintained at 0.8MPa, Feed inlet (II) is set at 3 / 4).
[0033] Pass into the 2,2-dimethoxypropane solution (the mol ratio of dehydrolinalool and phosphoric acid is 1:0.002) of the phosphoric acid of 0.242% from the feed port (II), flow is 0.039ml / min. The collected solution was a yellowish brown transparent liquid, and the gas phase analysis was performed on the material within 1 hour. The conversion rate of dehydrolinalool was 66.3%, and the selectivity to the product pseudoionone was 79.6%. The measurement results are listed in Table 1.
Embodiment 3-17
[0035]With reference to the preparation method of Example 1, change the conditions such as the type and amount of the SM reaction catalyst, the type and amount of the isomerization catalyst, the reaction temperature, the residence time, the molar equivalent ratio of dehydrolinalool and 2-methoxypropene in the reaction In addition, other reaction conditions and detection methods were the same as in Example 1, and pseudoionone was prepared.
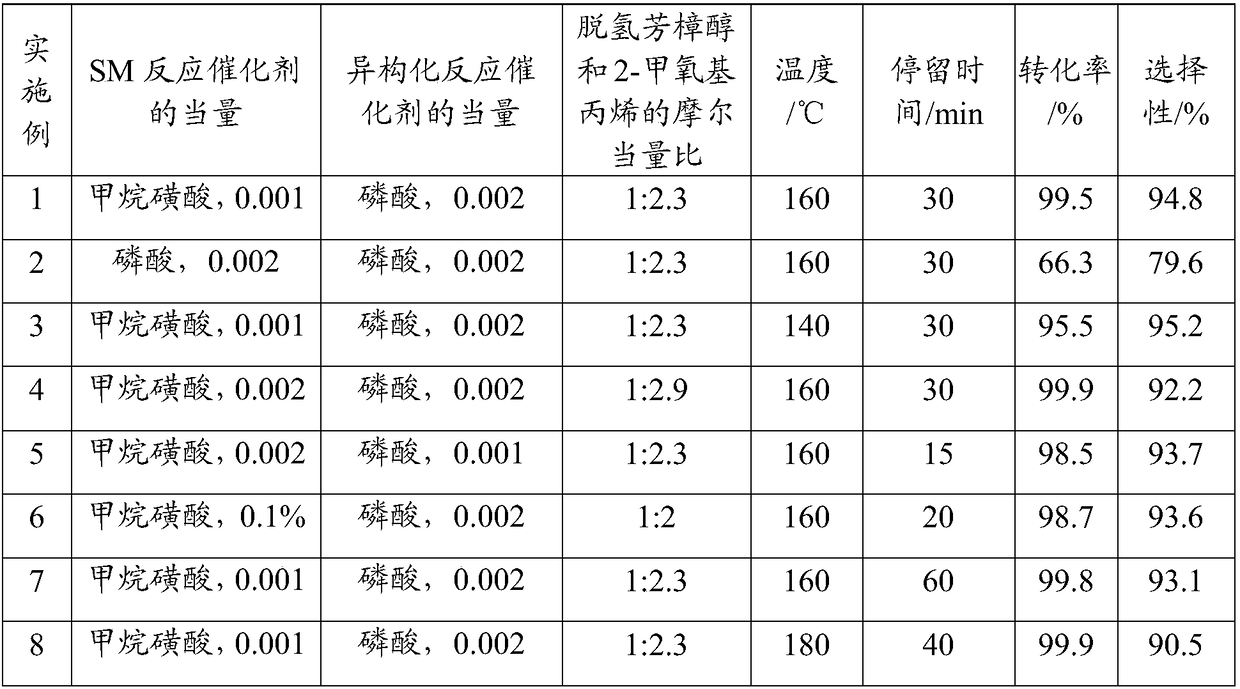
[0036] Catalyst equivalent is the ratio of the molar weight of catalyst and the molar weight of dehydrolinalool, and result is as shown in table 1:
[0037] Table 1 is the reaction condition and the result of embodiment 1-17
[0038]
[0039]
[0040] As can be seen from the test results of the examples in Table 1 above, in the examples that meet the conditions defined in the present application, the selectivity of the product and the conversion rate of the raw material are high, and the reaction can be completed quickly. In Example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com