Preparation method of algal polysaccharide derivatives
A technology of seaweed polysaccharide and derivatives is applied in the field of preparation of seaweed polysaccharide derivatives and achieves the effects of mild reaction conditions, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
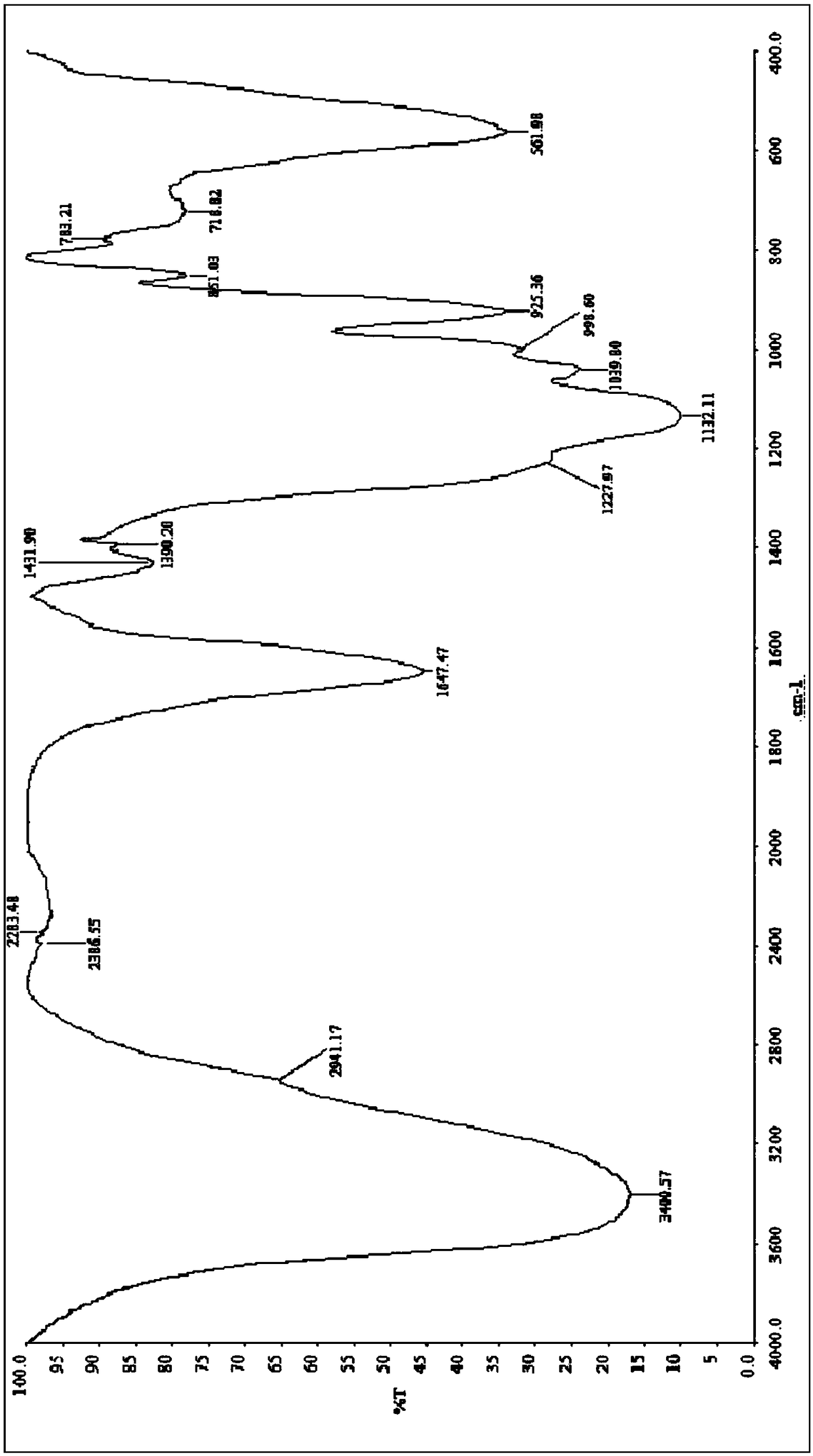
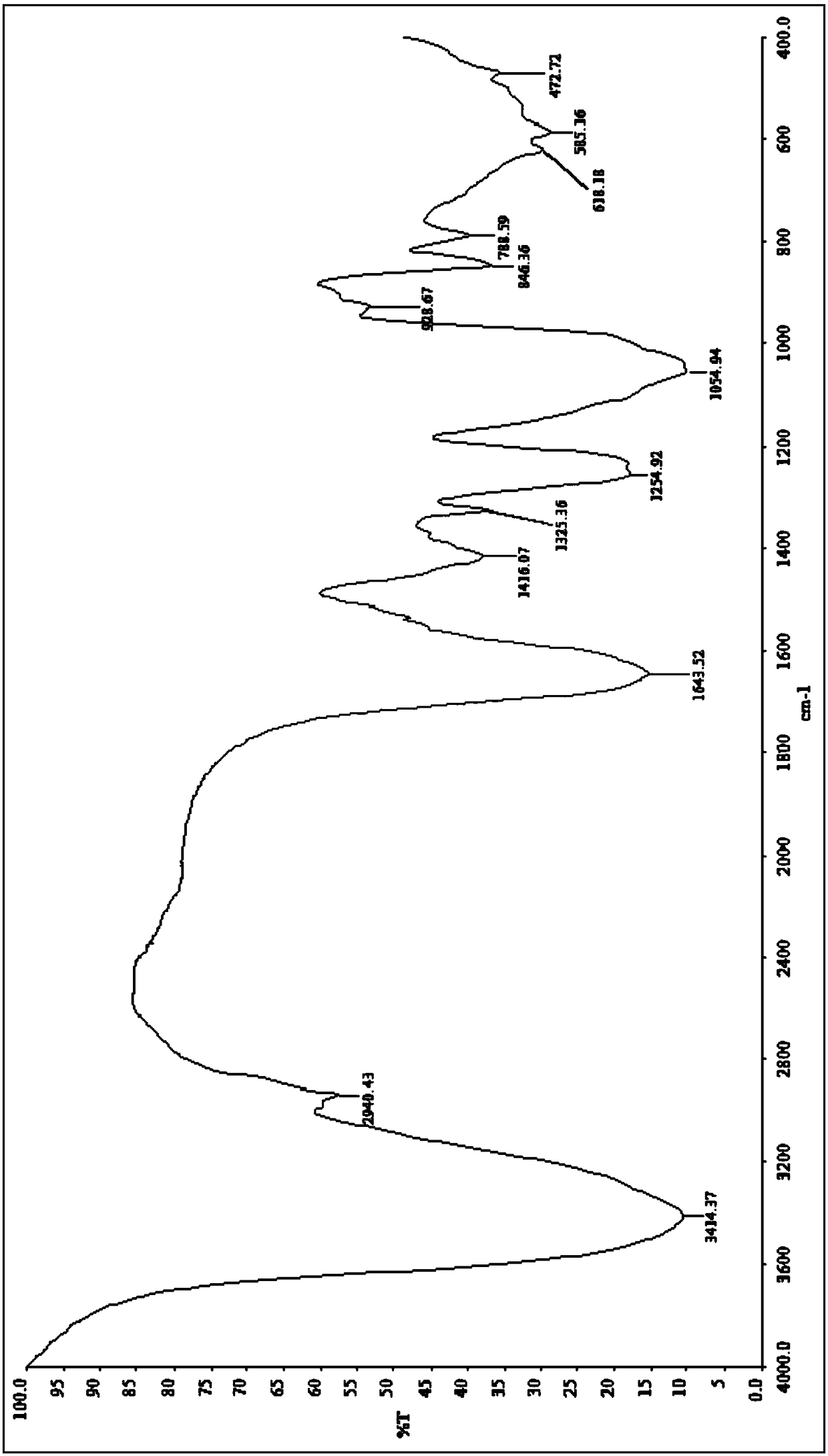
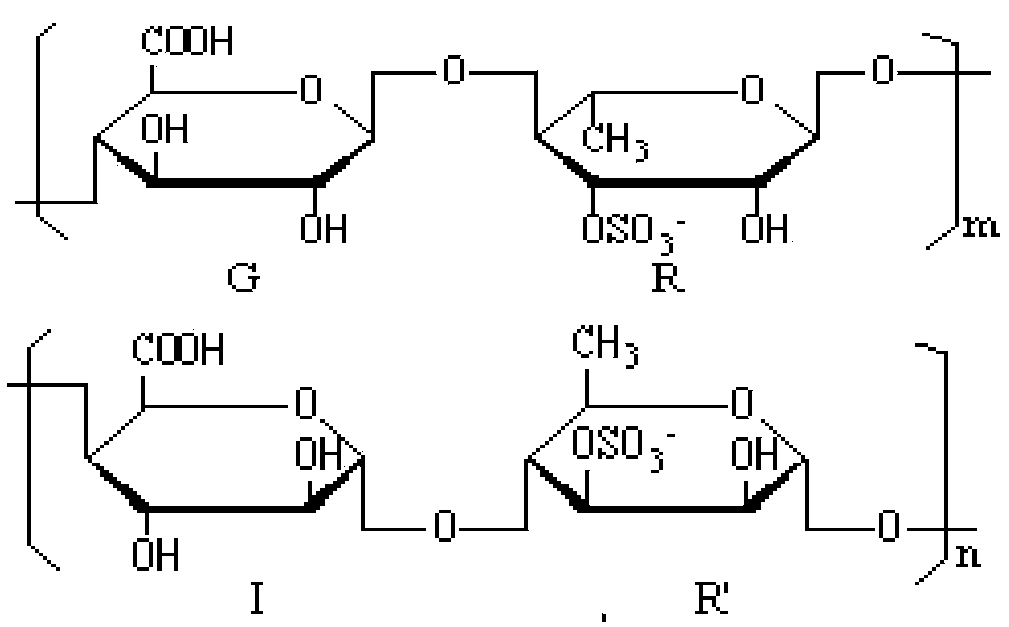
Image
Examples
Embodiment 1
[0031] Preparation of Ulva polysaccharides
[0032] (1) Pretreatment: Wash the Ulva algae body with tap water to remove silt, weeds and other attachments; then dry it naturally, pass through a 60-mesh sieve after crushing, and obtain the Ulva algae body powder, which is packaged for later use;
[0033] (2) Degreasing treatment: Weigh the Ulva pore algae body powder in step (1), reflux three times with 80% ethanol, centrifuge, and dry under reduced pressure at 40° C. to obtain degreasing Ulva pore algae body;
[0034] (3) Hot water extraction: extract the degreased Ulva pore algae bodies obtained in step (2) 3 times under the condition of a water bath at 125° C., extract 2 hours each time, filter, and combine the filtrates three times, then carry out rotary evaporation and concentration of the filtrates, and use 75% Precipitate with ethanol for 24h, filter out the precipitate;
[0035] (4) Drying: the precipitate filtered out in step (3) was dehydrated twice with absolute etha...
Embodiment 2
[0040] Preparation of Polysaccharide Derivatives from Ulva pore
[0041] (1) Ultrasonic treatment: add 1g of prepared Ulva polysaccharide into 100mL distilled water, add a certain amount of mixed phosphate reagent (STTP:STMP is 4:1), add 5g of anhydrous sodium sulfate, and use 200-400W power Ultrasonic treatment for 30 minutes to obtain a uniform mixed solution;
[0042] (2) pH adjustment: the mixed solution obtained in step (1) is adjusted to pH with newly prepared saturated sodium hydroxide solution or hydrochloric acid solution;
[0043] (3) Heating reaction: place the mixed solution after pH adjustment in step (2) in a three-necked flask, heat under reflux, and after heating for a certain period of time, cool to room temperature to obtain a reaction solution;
[0044] (4) Ethanol precipitation: Take the reaction solution in step (3), add 4 times the volume of the reaction solution with 95% ethanol and let it stand for 24 hours, remove the liquid, dry the precipitate, add ...
Embodiment 3
[0054] Determination of Hydroxyl Radical Scavenging of Phosphorylated Ulva Ulva Polysaccharide Derivatives
[0055] Take 150mmol / L sodium phosphate buffer solution (pH=7.4) 1mL, 360μg / mL saffron 1mL, 2mmol / LEDTA-Fe 0.5mL, add 1.0mL of the sample solution prepared according to the concentration of polysaccharide or polysaccharide derivative in Table 3, 3 %H 2 o 2 1 mL, shake well in a test tube, incubate and react in 37°C aqueous solution for 30 min, then measure the absorbance of the reaction system at 520 nm. Blank group: 1mL distilled water instead of the test sample. Control group: 1mL distilled water instead of the test sample, 1mL phosphate buffer instead of H 2 o 2 , the clearance results are shown in Table 3.
[0056] Calculation formula: clearance rate (%) = (A 样品 -A 空白 ) / (A 对照 -A 空白 )×100%
[0057] Table 3 Ulva pore polysaccharides and Ulva pore polysaccharide derivatives scavenging free radicals
[0058]
[0059] The experimental results show that, at d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com