Tumor microenvironment dual-response drug controlled-release microcapsule based on natural polysaccharide and application thereof
A tumor microenvironment and drug controlled release technology, applied in the field of biomedical polymer materials, can solve the problem of uncontrollable release rate and achieve the effect of cheap raw materials, strong stability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
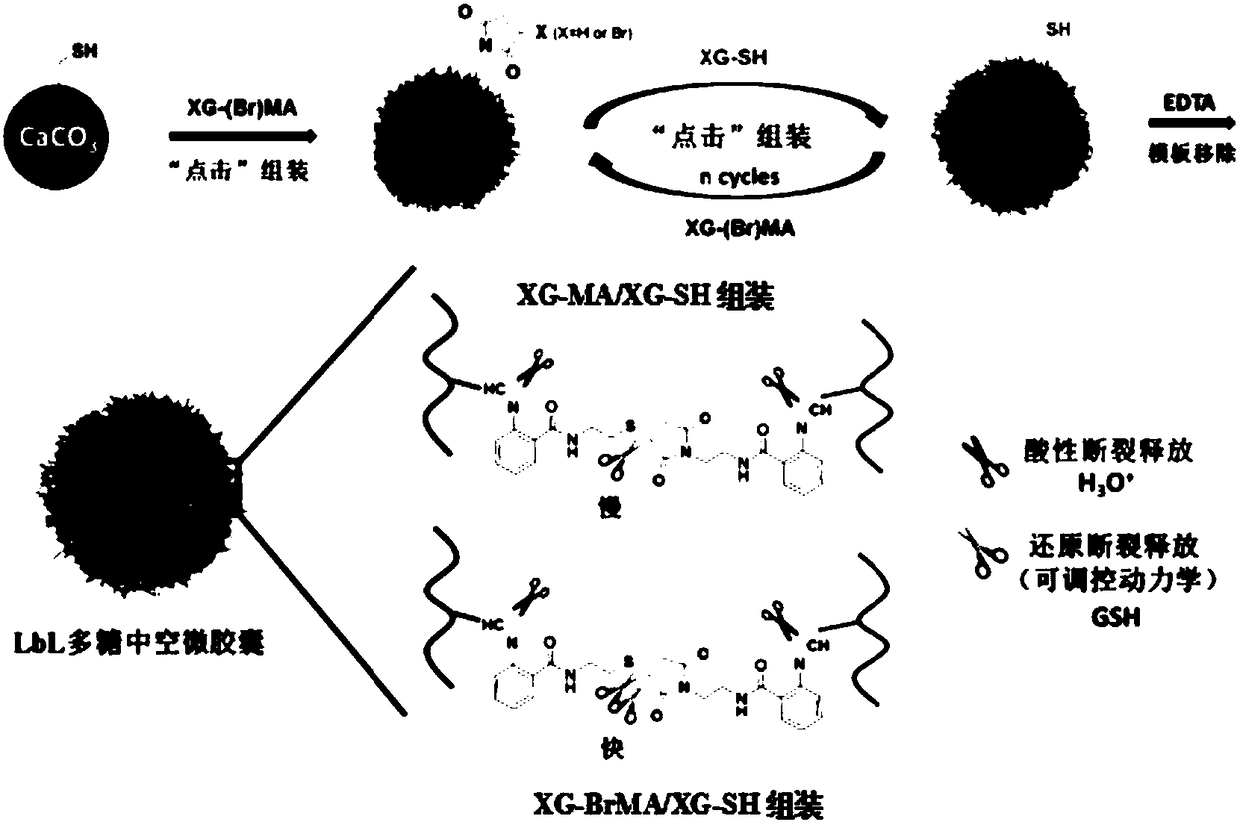
[0028] Using CaCO 3 Nanoparticles and XG-MA, XG-BrMA and XG-SH with functionalities of 20%, 50% and 40%, respectively. CaCO 3 Nanoparticles were dispersed into the chloroform solution of the anticancer drug, immersed for 15 minutes, centrifuged, the supernatant was removed, and the precipitate was dried for later use. Drug-loaded CaCO 3 The nanoparticles are placed in an aqueous solution of mercaptoethylamine hydrochloride, reacted for 25 minutes, and then centrifuged to obtain a thiol template loaded with anticancer drugs.
[0029] Place the thiolated template in the aqueous solution of XG-(Br)MA, vortex to disperse evenly, centrifuge after 10 minutes of reaction, remove the supernatant, wash with deionized water for 3 times, and then place the microspheres in XG-SH In the aqueous solution, the second layer was deposited by the same method, the microspheres were washed twice with deionized water, and then the above LbL assembly process was repeated, and XG-(Br)MA and XG-SH...
Embodiment 2
[0032] Using CaCO 3 Nanoparticles and XG-MA, XG-BrMA and XG-SH with functionalities of 30%, 50% and 30%, respectively. CaCO 3 Nanoparticles were dispersed into the chloroform solution of anticancer drugs, immersed for 20 minutes, centrifuged, the supernatant was removed, and the precipitate was dried for later use. Drug-loaded CaCO 3 The nanoparticles are placed in an aqueous solution of mercaptoethylamine hydrochloride, reacted for 15 minutes, and then centrifuged to obtain a thiol template loaded with anticancer drugs.
[0033] Place the thiol-modified microspheres in the aqueous solution of XG-(Br)MA, vortex to disperse evenly, centrifuge after 25 minutes of reaction, remove the supernatant, wash twice with deionized water, and continue to place the microspheres in the thiol In the aqueous solution of the xyloglucan derivative (XG-SH) of the group, the second layer was deposited by the same method, the microspheres were washed with deionized water for 4 times, and the ab...
Embodiment 3
[0036] Using CaCO 3Nanoparticles and XG-MA, XG-BrMA and XG-SH with functionalities of 50%, 10% and 60%, respectively. CaCO 3 Nanoparticles were dispersed into the chloroform solution of anticancer drugs, immersed for 30 minutes, centrifuged, the supernatant was removed, and the precipitate was dried for later use. Drug-loaded CaCO 3 The nanoparticles were placed in an aqueous solution of mercaptoethylamine hydrochloride, reacted for 25 minutes, and then centrifuged. Obtain sulfhydryl templates loaded with anticancer drugs.
[0037] Place the thiol-modified microspheres in an aqueous solution of XG-(Br)MA, vortex to disperse evenly, centrifuge after 10 minutes of reaction, remove the supernatant, wash with deionized water for 4 times, and then continue to place the microspheres in the thiol In the aqueous solution of the xyloglucan derivative (XG-SH) of the group, the second layer was deposited by the same method, the microspheres were washed with deionized water for 3 time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com