A polyketene compound, its application as a dual-fluorescent emission organic light-emitting material and its preparation method
A technology for luminescent materials and compounds, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult industrialized production, difficult product separation, complex synthesis routes, etc., to promote solid-state luminescence performance, reduce mutual effect, the effect of enhancing the luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
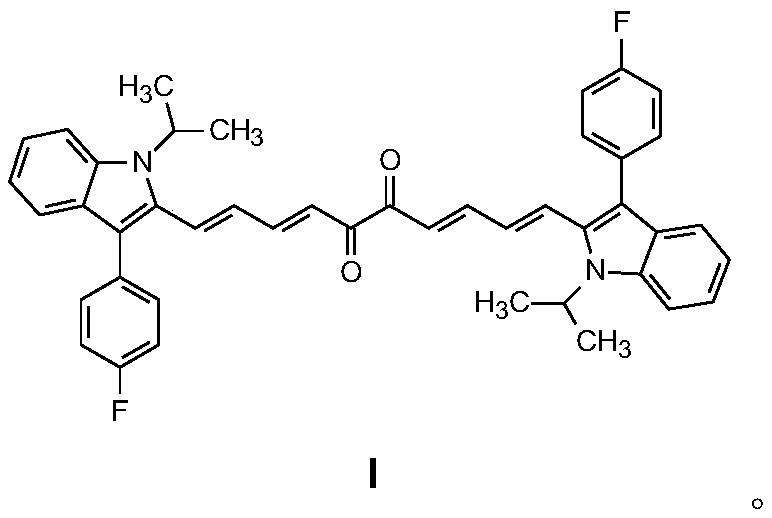
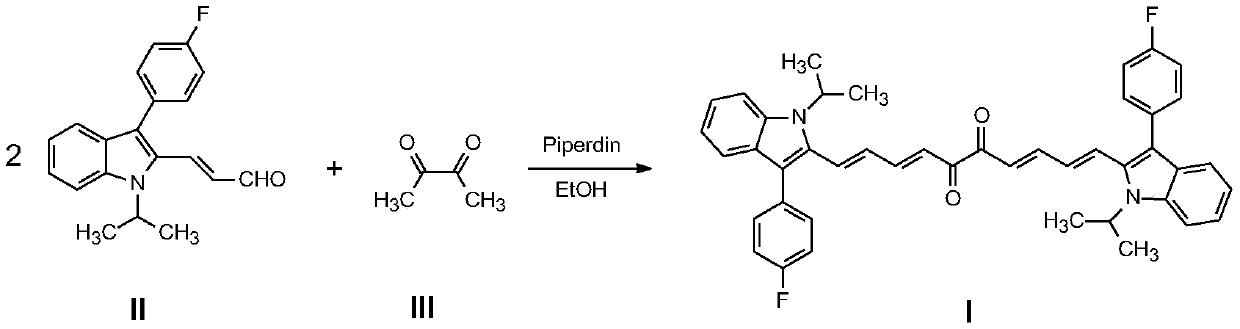
[0027] Example 1: 1,10-bis(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)-1,3,7,9-decatetraene-5, Preparation of 6-diketone (I):
[0028] In a dry 250mL round bottom flask, add 2,3-butanedione (0.01mol), 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl ) acrolein (0.02mol), and anhydrous methanol (150mL), then add piperidine (0.5mL) to the solution under rapid stirring, stir and reflux for 9 hours, cool to room temperature after the reaction, and separate out a brown solid substance , filtered under reduced pressure, washed twice with absolute ethanol to obtain the crude product, the crude product was recrystallized with ethanol-acetone mixed solvent (volume ratio of ethanol and acetone is 3:1), dried in vacuum to obtain brown solid 1,10- bis(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)-1,3,7,9-decatetraene-5,6-dione. The yield was 18%.
[0029] The obtained product was determined to be the target product by proton nuclear magnetic resonance spectrum and carbon nuclear magne...
Embodiment 2
[0031] Example 2: 1,10-bis(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)-1,3,7,9-decatetraene-5, Preparation of 6-diketone (I):
[0032] In a dry 250mL round bottom flask, add 2,3-butanedione (0.01mol), 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl ) acrolein (0.022mol), and absolute ethanol (150mL), then add piperidine (0.5mL) to the solution under rapid stirring, stir and reflux for 5 hours, cool to room temperature after the reaction, and separate out a brown solid substance , filtered under reduced pressure, washed twice with anhydrous methanol to obtain the crude product, the crude product was recrystallized with ethanol-acetone mixed solvent (the volume ratio of ethanol and acetone was 3:1), and dried in vacuo to obtain a brown solid 1,10- bis(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)-1,3,7,9-decatetraene-5,6-dione. The yield was 19%.
Embodiment 3
[0033] Embodiment 3: Solid-state luminescent performance test
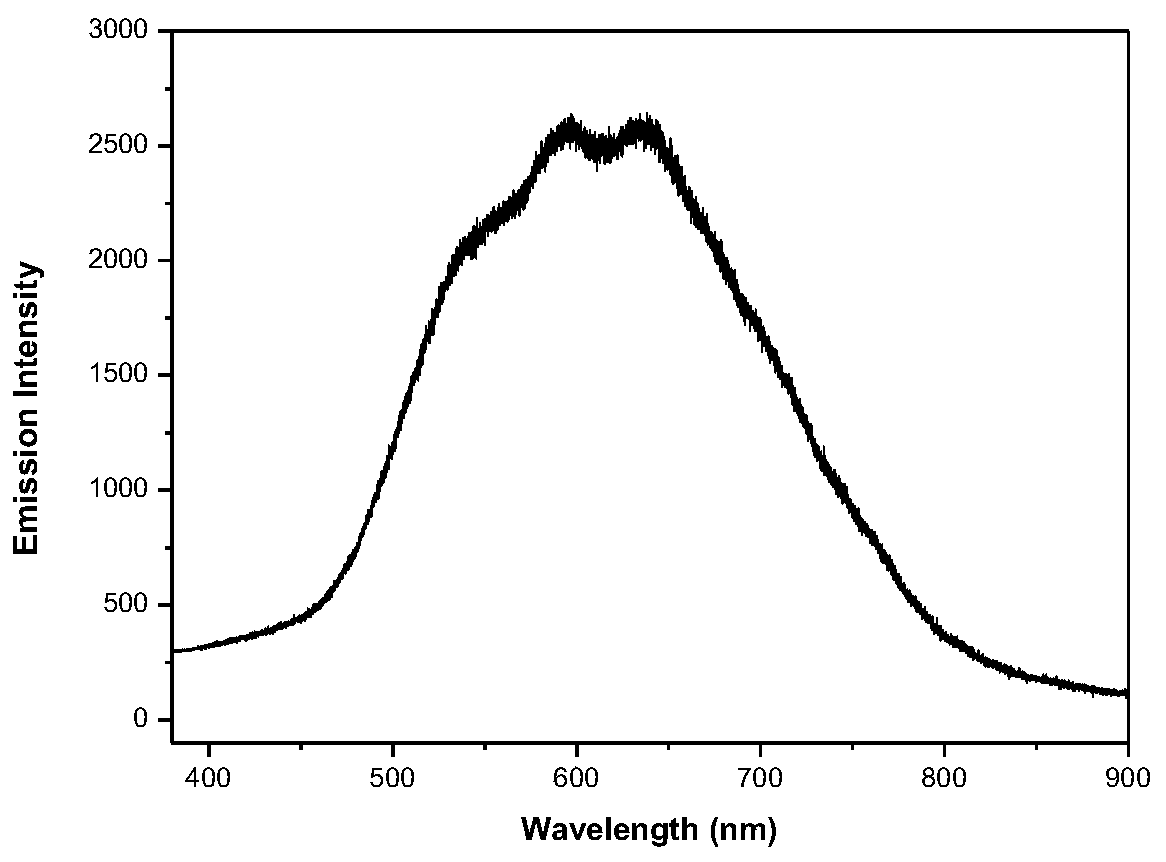
[0034] The solid-state photoluminescence spectrum is measured with a Horiba Jobin-Yvon LabRam HR800 laser Raman spectrometer, the excitation light source is a 325nm He–Cd laser, and the polyketene compound prepared in Example 1 is used for solid-state luminescence performance testing. The test results are shown in figure 1 .
[0035] Depend on figure 1It can be seen that the polyketene compound obtained in Example 1 exhibits dual fluorescence emission characteristics under 325nm laser excitation, and its emission spectrum covers almost the entire visible region, and is accompanied by a shoulder structure in the short-wave region. The emission wavelengths of its dual emission peaks are 597nm and 638nm respectively, and the maximum emission wavelength is 638nm. Therefore, the molecule emits strong red fluorescence.
[0036] The above results show that the polyketene compound, as a dual-fluorescence emission organi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com