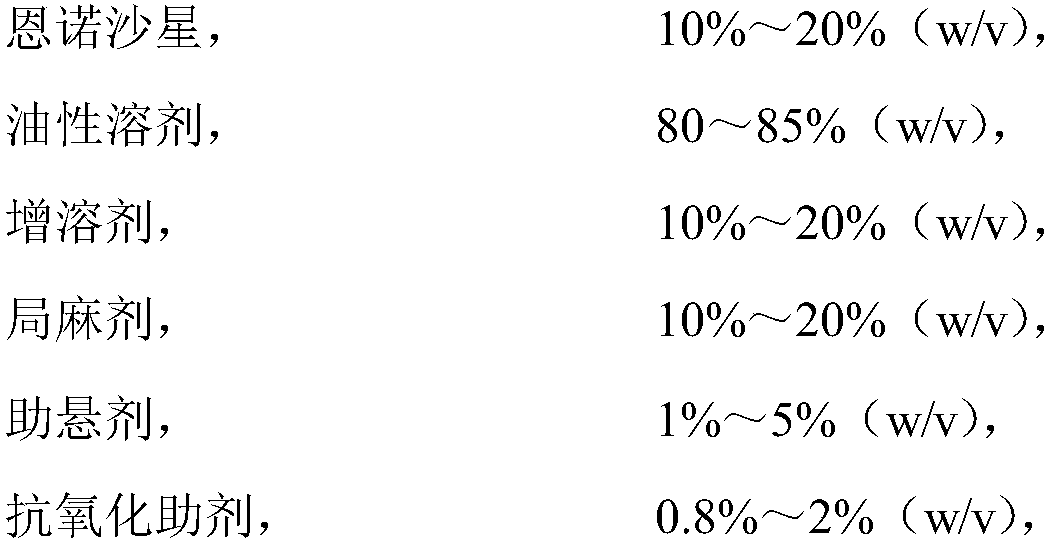Non-aqueous solvent long-acting enrofloxacin injection liquid and preparation process thereof
A technology of enrofloxacin and non-aqueous solvent, applied in the field of medicinal chemistry, can solve the problems of large stress reaction of animals, poor effect, time period of drug effect, etc., and achieve the effects of long action time, good preservation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The invention provides a non-aqueous solvent long-acting enrofloxacin injection, as follows: the formula of the injection is expressed in terms of mass concentration:
[0025]
[0026]
[0027] Among them, the oily solvent is made by mixing ethyl oleate, coix seed oil, and cottonseed oil, and is prepared according to the following ratio in turn: 5:2.5:1.8;
[0028] The co-solvent is prepared by mixing lactic acid, propylene glycol methyl ether, polyvinyl alcohol, and ox bile in a sequential ratio of 10:3.5:0.8:1;
[0029] The local anesthetic is prepared and mixed with benzyl alcohol and benzylamine at a ratio of 3:0.8;
[0030] The blocker is prepared by mixing aluminum stearate, sodium alginate, and glycerin in a ratio of 6:1:1.5 in sequence;
[0031] Antioxidant features Vitamin E.
[0032] The above-mentioned non-aqueous solvent long-acting enrofloxacin injection is obtained through the following preparation process:
[0033] Step A, adding the prescribed a...
Embodiment 2
[0036] The invention provides a non-aqueous solvent long-acting enrofloxacin injection, as follows: the formula of the injection is expressed in terms of mass concentration:
[0037]
[0038] Among them, the oily solvent is made by mixing ethyl oleate, coix seed oil, and cottonseed oil, and is prepared according to the following ratio in turn: 5:2.5:1.8;
[0039] The co-solvent is prepared by mixing lactic acid, propylene glycol methyl ether, polyvinyl alcohol, and ox bile in a sequential ratio of 10:3.5:0.8:1;
[0040] The local anesthetic is prepared and mixed with benzyl alcohol and benzylamine at a ratio of 3:0.8;
[0041] The blocker is prepared by mixing aluminum stearate, sodium alginate, and glycerin in a ratio of 6:1:1.5 in sequence;
[0042] Antioxidant features Vitamin E.
[0043] The above-mentioned non-aqueous solvent long-acting enrofloxacin injection is obtained through the following preparation process:
[0044] Step A, adding the prescribed amount of oil...
Embodiment 3
[0047] The invention provides a non-aqueous solvent long-acting enrofloxacin injection, as follows: the formula of the injection is expressed in terms of mass concentration:
[0048]
[0049] Among them, the oily solvent is made by mixing ethyl oleate, coix seed oil, and cottonseed oil, and is prepared according to the following ratio in turn: 5:2.5:1.8;
[0050] The co-solvent is prepared by mixing lactic acid, propylene glycol methyl ether, polyvinyl alcohol, and ox bile in a sequential ratio of 10:3.5:0.8:1;
[0051] The local anesthetic is prepared and mixed with benzyl alcohol and benzylamine at a ratio of 3:0.8;
[0052] The blocker is prepared by mixing aluminum stearate, sodium alginate, and glycerin in a ratio of 6:1:1.5 in sequence;
[0053] Antioxidant features Vitamin E.
[0054] The above-mentioned non-aqueous solvent long-acting enrofloxacin injection is obtained through the following preparation process:
[0055] Step A, adding the prescribed amount of oil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com