A kind of preparation method of difluorophosphoric acid and lithium difluorophosphate
A technology of lithium difluorophosphate and difluorophosphoric acid, which is applied in the direction of phosphorus oxyacids, chemical instruments and methods, phosphorus compounds, etc., can solve the problems of low raw material utilization rate and high production cost, and achieve low cost, small side reactions, The effect of a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
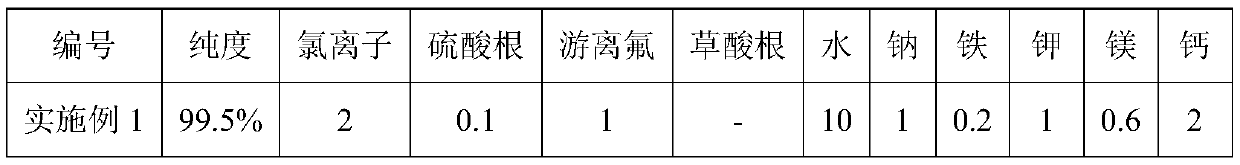
Examples
Embodiment 1
[0022] The preparation method of the lithium difluorophosphate of the present embodiment adopts the following steps:
[0023] 1) Dissolve 252g of pyrophosphoryl chloride in 500g of dichloromethane, cool to -20°C, then dropwise add 18g of high-purity water, stir and react at -20°C for 8 hours, remove the dichloromethane by distillation under reduced pressure, and obtain 266.49g of dichlorophosphoric acid, The hydrolysis yield is 98.7%.
[0024] 2) Take 200 g of dichlorophosphoric acid, add 168.6 g of zinc fluoride, fully stir the reaction at 80°C for 24 hours, distill under reduced pressure, collect the distillate to obtain 148 g of difluorophosphoric acid, the yield is 98%;
[0025] Dissolve 148g of difluorophosphoric acid in 2 times (by volume) of ethylene glycol dimethyl ether, then cool to 0°C, add 55g of lithium carbonate, heat up to 30°C, fully react for 10h, filter off the excess lithium carbonate to obtain The clear and transparent solution was concentrated under reduc...
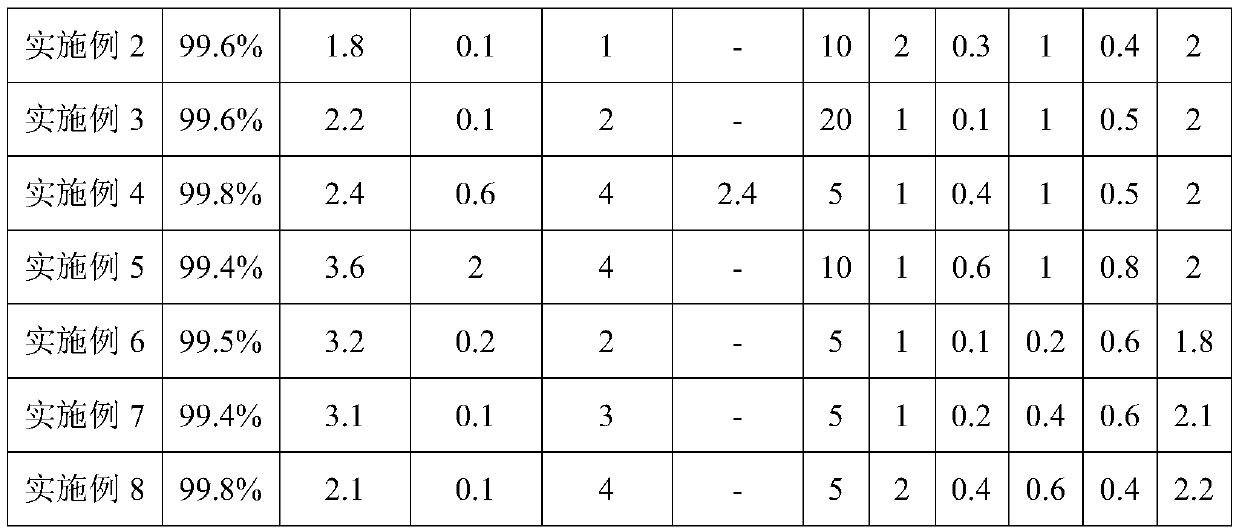
Embodiment 2
[0027] The preparation method of the lithium difluorophosphate of the present embodiment adopts the following steps:
[0028] 1) Adopt the method for embodiment 1 to prepare dichlorophosphoric acid.
[0029] 2) Take 280g of dichlorophosphoric acid, add 60g of anhydrous hydrogen fluoride (HF), fully stir the reaction at 10°C for 20h, blow out the excess anhydrous hydrogen fluoride with dry nitrogen, then distill under reduced pressure, collect the distillate to obtain difluoride Phosphoric acid 206g, yield is 98.2%;
[0030] Dissolve 200g of difluorophosphoric acid into twice (by volume) ethylene glycol dimethyl ether, then cool to 0°C, add 83.3g of lithium chloride (LiCl), raise the temperature to 40°C, fully react for 8 hours, and decompress Ethylene glycol dimethyl ether was concentrated and recovered, and the crystallized product was crude lithium difluorophosphate. After recrystallization using ethylene glycol dimethyl ether solvent, vacuum drying at 80°C for 12 hours gav...
Embodiment 3
[0032] The preparation method of the lithium difluorophosphate of the present embodiment adopts the following steps:
[0033] 1) Adopt the method for embodiment 1 to prepare dichlorophosphoric acid.
[0034] 2) Take 272g of dichlorophosphoric acid, add bismuth trifluoride (BiF 3 ) 358g, fully stirred and reacted at 60°C for 36h, distilled under reduced pressure, collected distillate to obtain 201.1g of difluorophosphoric acid, the yield was 98.6%;
[0035] Dissolve 200g of difluorophosphoric acid in twice (by volume) ethylene glycol dimethyl ether, then cool to 0°C, add 82.4g of lithium hydroxide (LiOH·H 2 O), heated up to 50°C, fully reacted for 4 hours, concentrated under reduced pressure to recover ethylene glycol dimethyl ether, and the crystallized product was crude lithium difluorophosphate. After the crude product lithium difluorophosphate was vacuum-dried at 80°C, the After recrystallization from alcohol dimethyl ether solvent, vacuum drying was carried out for 8 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com