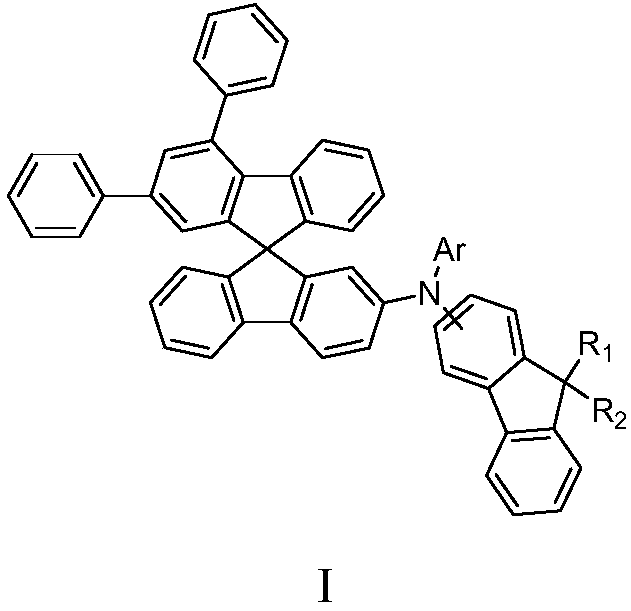
Fluorene-containing triarylamine compound as well as application and light-emitting device thereof
A technology of triaromatic amines and compounds, applied in the field of organic electroluminescent materials, can solve the problems of high preparation energy consumption, affect device efficiency, poor thermal stability, etc., and achieve high luminous purity, high luminous efficiency, and good thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
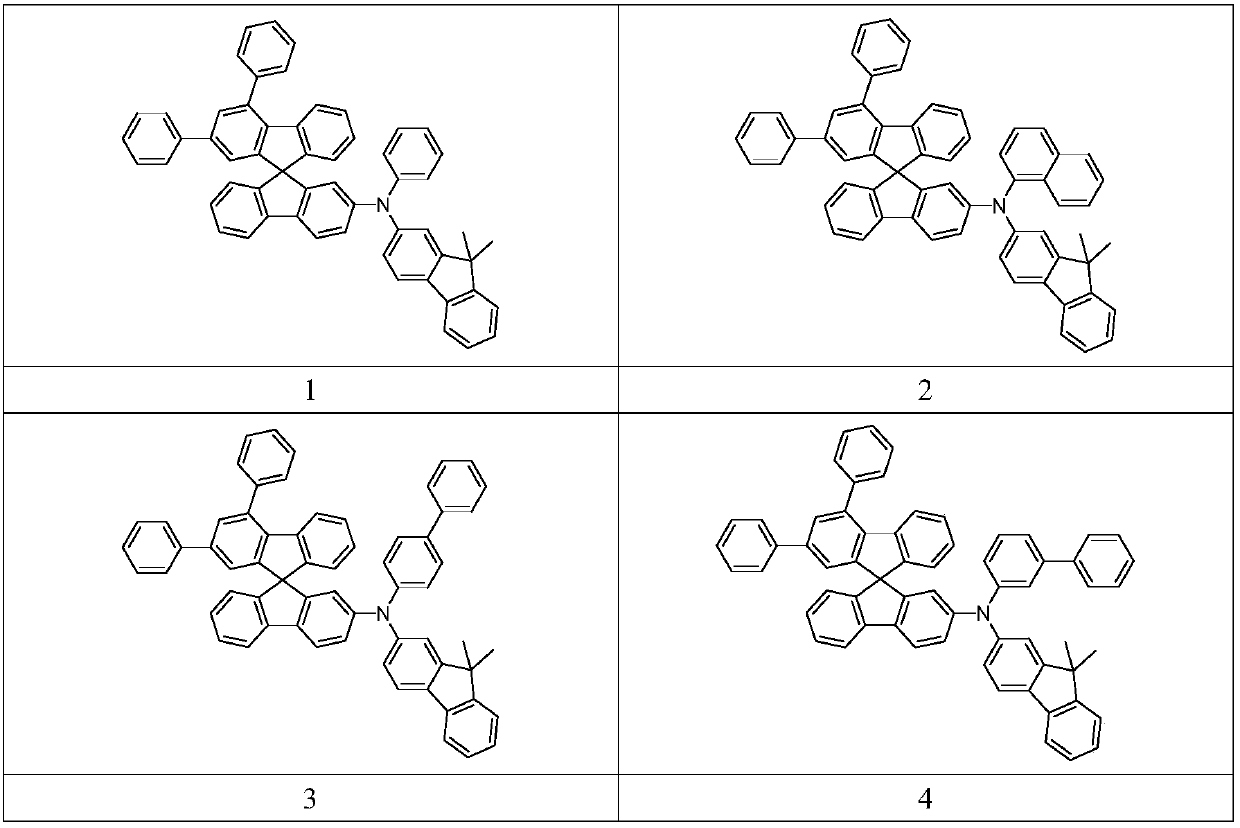
[0048] Synthetic route of compound 3
[0049]
[0050] The synthetic method of intermediate 3-1
[0051] In the flask, add 2,4-diphenylphenylboronic acid (25g, 91mmol), o-bromoiodobenzene (25.6g, 91mmol), potassium carbonate (25g, 182mmol), tetrakistriphenylphosphine palladium (0.5g), Tetrahydrofuran (250 mL) and water (100 mL) were heated to reflux for 12 hours under the protection of nitrogen, cooled, extracted with dichloromethane, dried, concentrated, and the crude product was purified by column chromatography to obtain 18.9 g of product with a yield of 54%.
[0052] The synthetic method of intermediate 3-2
[0053] In the flask, add intermediate 3-1 (15g, 39mmol), anhydrous tetrahydrofuran (150mL), cool to -78 degrees under nitrogen protection, slowly add 2.5M n-butyllithium solution (15.6mL, 39mmol), Stir for 2 hours, then slowly add this solution to a tetrahydrofuran solution dissolved in 2-bromofluorenone (10g, 39mmol), react for 5 hours, then slowly add 1N dilute...
Embodiment 21-40
[0065] Fabrication of Organic Electroluminescent Devices
[0066] Preparation of OLEDs using the compounds of the Examples
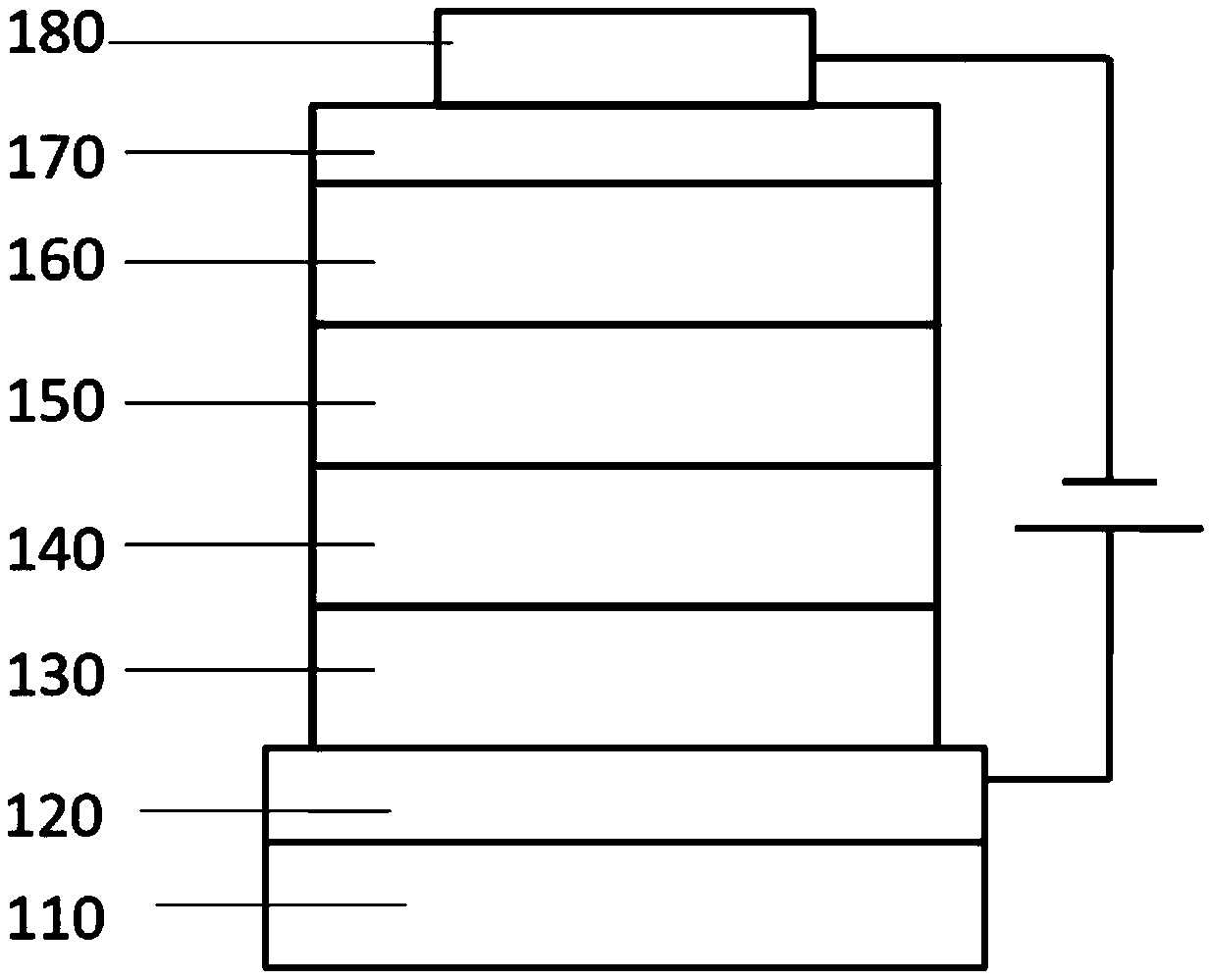
[0067] First, the transparent conductive ITO glass substrate 110 (with anode 120 on it) (China CSG Group Co., Ltd.) was washed in sequence with deionized water, ethanol, acetone and deionized water, and then treated with oxygen plasma for 30 seconds.
[0068] Then, PEDOT:PSS (polyethylenedioxythiophene-poly(styrene sulfonate)) with a thickness of 45 nm was spin-coated on the ITO as the hole injection layer 130, and dried at 150° C. for 30 minutes.
[0069] Then, the compound of the present invention was vapor-deposited as the hole transport material 140 on the hole injection layer to a thickness of 40 nm.
[0070] Then, a luminescent layer 150 with a thickness of 30nm is evaporated on the electron blocking layer, wherein CBP is the host luminescent material, and the weight ratio is 8% of Ir(ppy) 3 As a phosphorescent doping guest material.
[0071] Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com