4-aza-aryl alkanol compound and synthetic method thereof
A technology for azaarylalkane and alcohol compounds, which is applied in the field of synthesis of 4-azaarylalkanol compounds, can solve the problem of not being able to meet the needs for the diverse synthesis of complex alcohol derivatives in synthetic chemistry, difficulty in selectivity, and uniform splitting. Difficulty responding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088]
[0089] Under nitrogen protection, add heteroaryl compound 1a (0.4mmol), alcohol 2a (x mmol) and organic solvent (2.0mL) into the reaction flask, stir to dissolve, then add hypervalent aryl iodide compound Ar-I to the mixed solution III (y mmol), irradiate the reaction vial with light of specific power and wavelength (hv) and keep it at room temperature, or place the reaction vial in a dark room to heat. After the reaction is complete, add aq.KOH until pH>8 to quench the reaction , extracted with ethyl acetate (3x 10mL), combined the organic phases, washed with saturated brine, dried and filtered over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product, which was separated and purified by ethyl acetate / petroleum ether silica gel column chromatography to obtain compound 3a.
[0090]
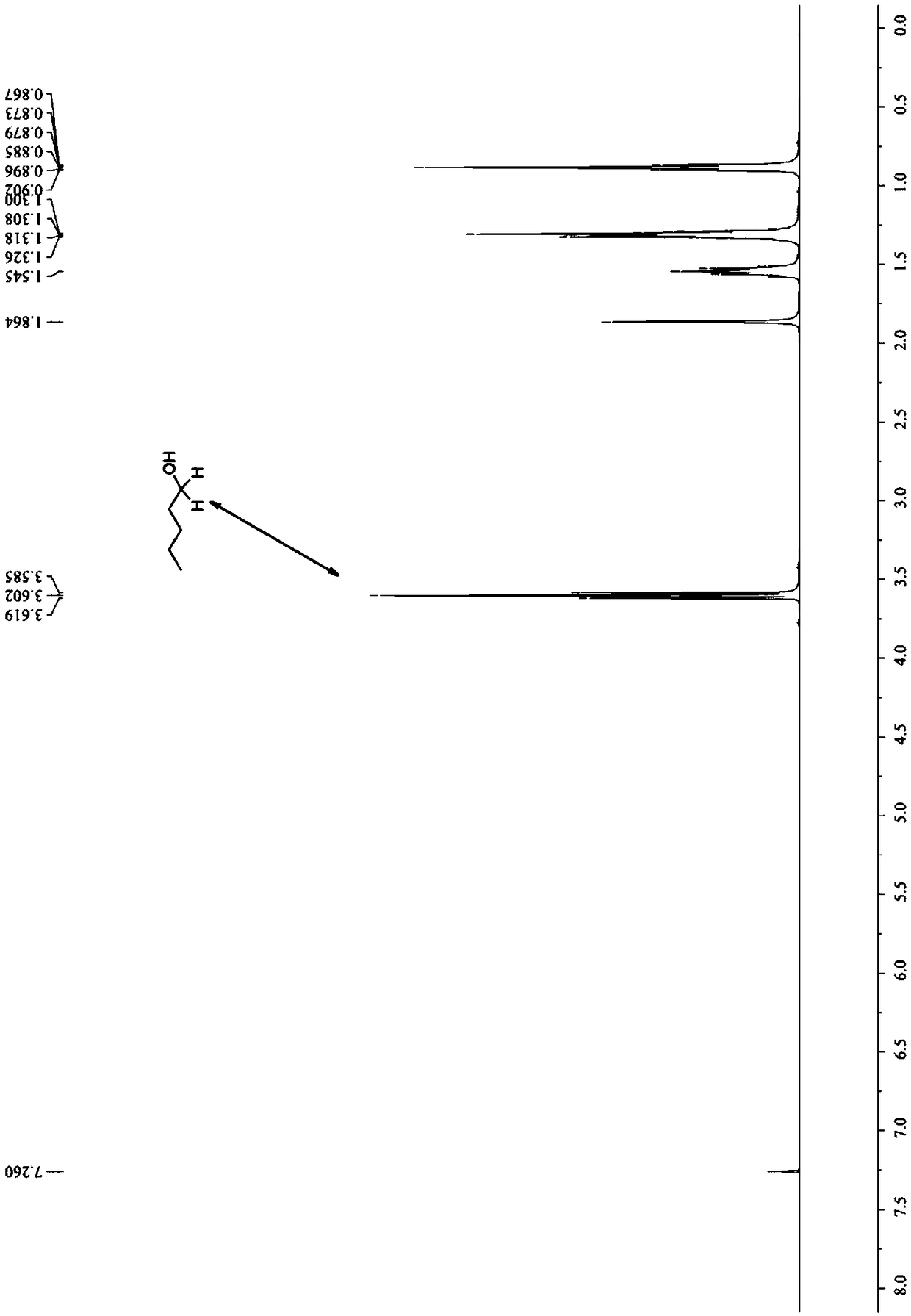
[0091] 3a: yellow or colorless oily liquid, 1 H NMR (400MHz, CDCl 3 )δ8.03(d,J=8.4Hz,1H),7.92(dd,J=8.0,0.8Hz,1H),7.64(ddd,J=8.0,6.8,1.2Hz,1H)...
Embodiment 2
[0095]
[0096] Under the protection of inert gas, add heteroaryl compound 1b (0.4mmol), alcohol 2a (2.0mmol) and dichloromethane DCM (2.0mL) into the reaction flask, stir to dissolve, then add PIFA (0.92mmol) to the mixed solution , place the reaction bottle under 100W blue LED light, and use a fan to maintain room temperature. After the reaction is complete, add aq.KOH until pH>8 to quench the reaction, extract with ethyl acetate (3x 10mL), combine the organic phases, and saturate Washed with brine, dried and filtered over anhydrous sodium sulfate, concentrated under reduced pressure, separated and purified by ethyl acetate / petroleum ether silica gel column chromatography to obtain compound 3b.
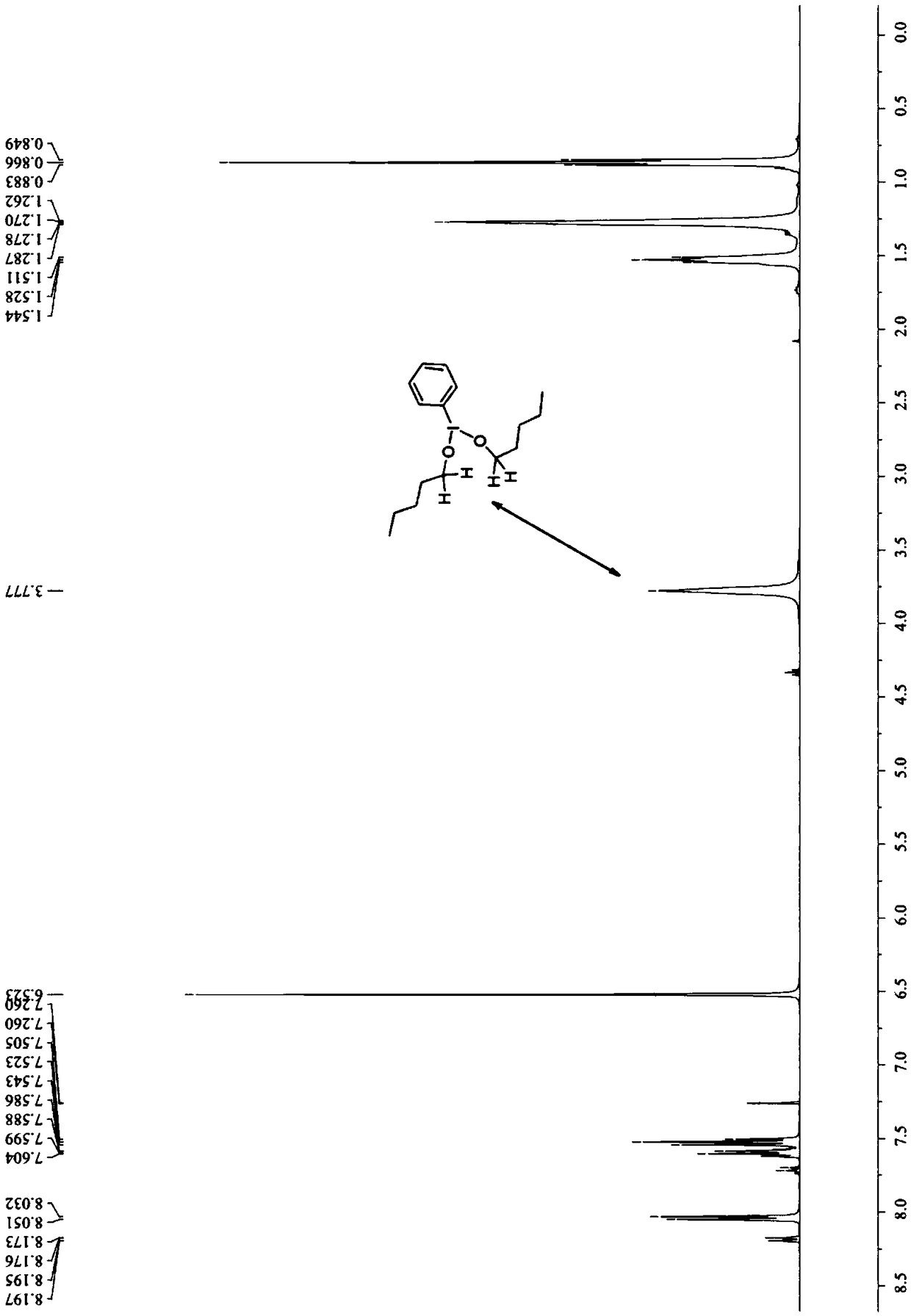
[0097] 3b: yellow oily liquid, 1 H NMR (400MHz, CDCl 3 )δ8.12(dd, J=8.4,0.8Hz,1H),8.01(d,J=8.4Hz,1H),7.67(ddd,J=8.4,6.8,1.2Hz,1H),7.52(ddd,J =8.0,6.8,0.8Hz,1H),7.36(s,1H),3.59(t,J=6.4Hz,2H),3.33(s,1H),3.11-3.01(m,1H),1.92-1.82( m,1H),1.77-1.67(m,1H),1.64-1.53(m,1H),1.50-1.39(m...
Embodiment 3
[0099]
[0100] The difference between this example and Example 2 is that 1b in Example 2 was replaced by 1c, and the rest of the operation was basically the same as that of Example 2, and compound 3c was obtained after reaction and purification.
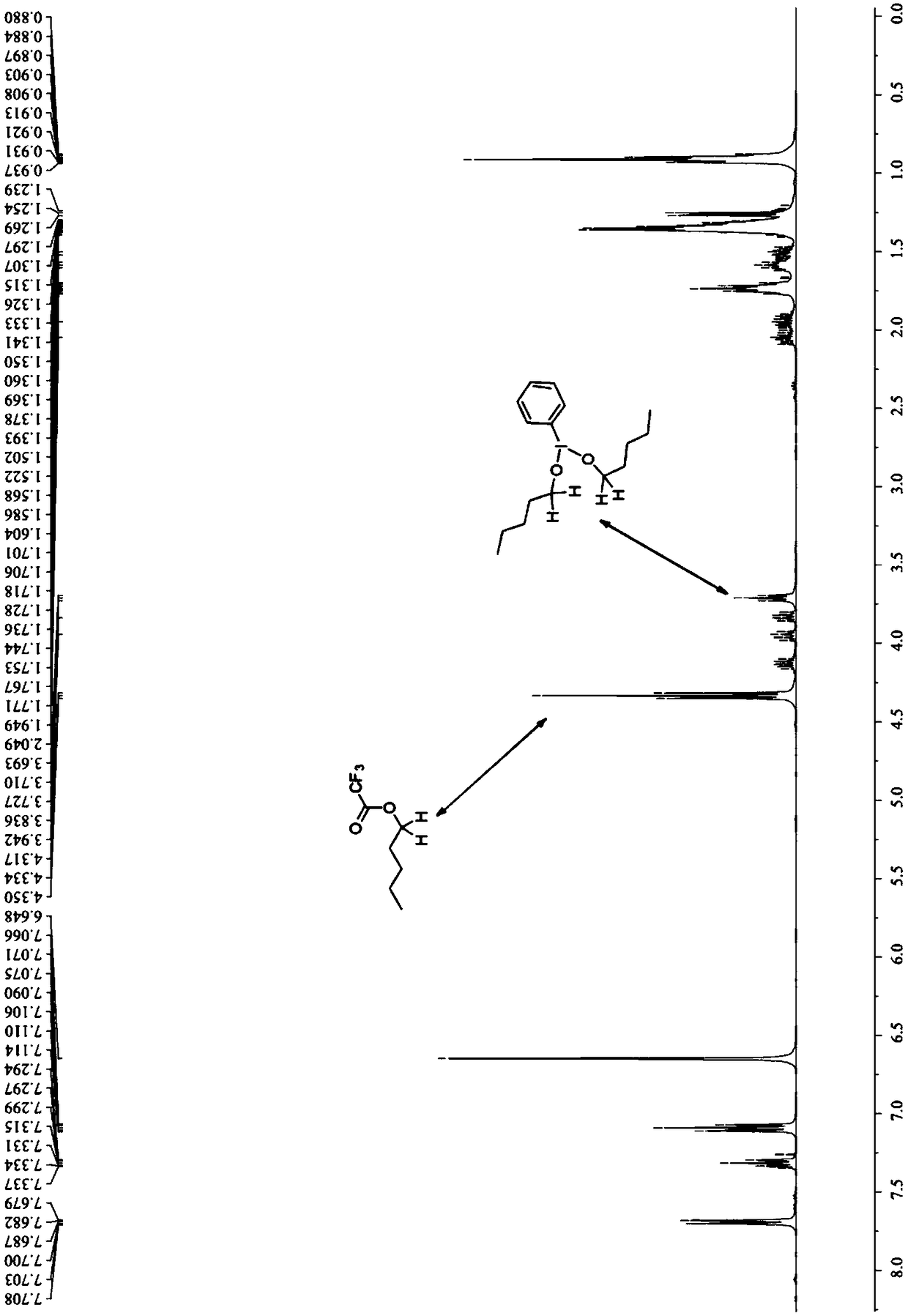
[0101] 3c: yellow oily liquid, 1 H NMR (400MHz, CDCl 3 )δ8.01-7.95(m,2H),7.58(ddd,J=8.4,6.8,1.2Hz,1H),7.41(ddd,J=8.0,6.8,0.8Hz,1H),7.11(s,1H) ,3.68(br,1H),3.60(t,J=6.4Hz,2H),3.57-3.46(m,1H),2.63(s,3H),1.88-1.66(m,2H),1.64-1.43(m ,2H),1.30(d,J=6.8Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ158.1,153.2,147.4,128.6,128.5,125.0,122.3,118.0,61.7,33.0,32.6,30.2,24.7,20.7.FT-IR:ν(cm -1 ) 2963, 2934, 2862, 1598, 1562, 1509, 1458, 1415. HRMS [ESI] calcd for C 15 h 20 NO[M+H] + 230.1539, found 230.1536.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com