Expression purification method for HBcAg
An expression, purification and inducible expression technology, applied in the field of HBcAg expression and purification, can solve the problems of low expression amount and complicated purification method, and achieve the effect of simple steps, good antigenicity, and satisfying detection requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
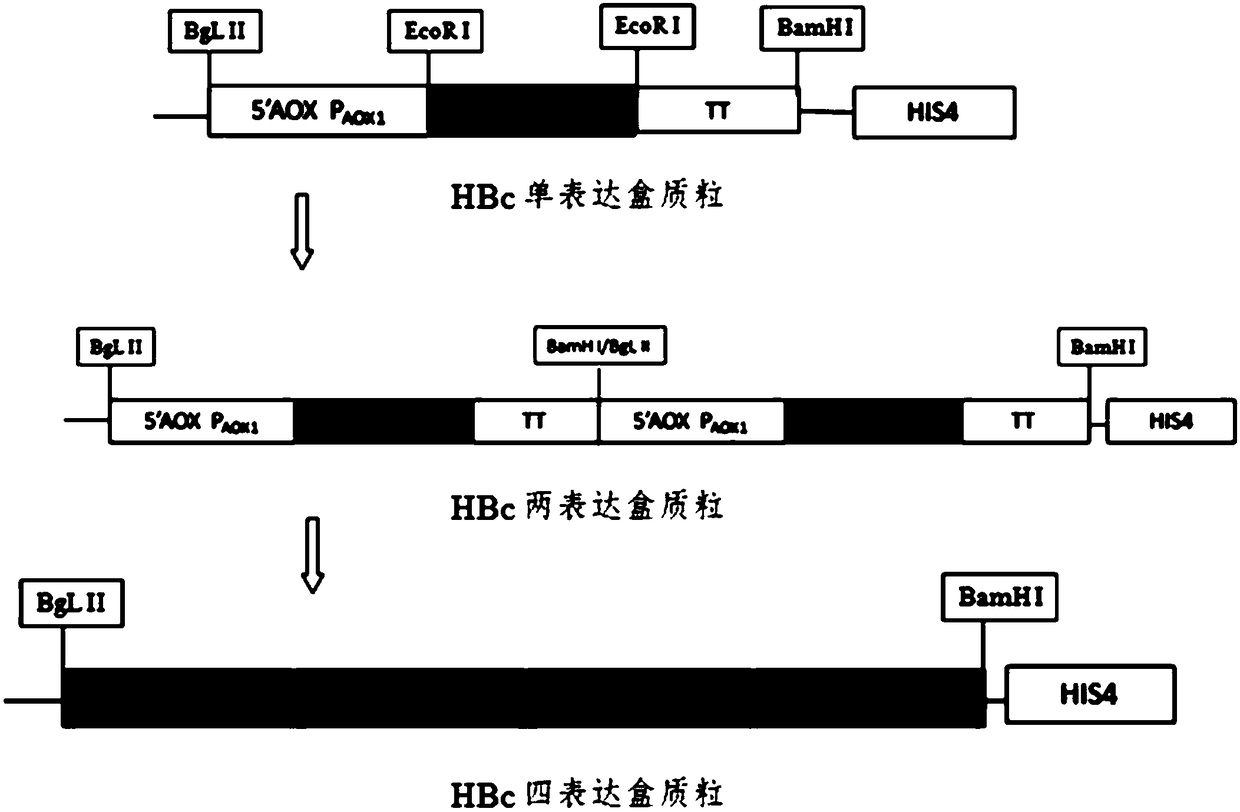
[0060] Construction of HBc yeast multi-copy vector
[0061] The full-length HBc gene is obtained by codon optimization according to the C-terminal arginine-rich region of the gene C-type sequence published by NCBI. The sequence is shown in SEQ ID NO: 1. The whole gene fragment is chemically synthesized and then amplified by PCR. Gene.
[0062] The primers used in PCR amplification are:
[0063] N-terminal primer:
[0064] CCAATgaattcGCCACCATGGACATTGACCCGTATAAAG;
[0065] C-terminal primer:
[0066] CAATgaattcTTAACACTGGCTTTCGCGAGAC.
[0067] The HBcAg gene of the present invention is the domestic popular strain sequence (KR013930.1) found on NCBI, GCCACC is added to the N-terminus to facilitate the correct initiation of translation of the target gene, and codon optimization is performed at the C-terminus to facilitate protein expression.
[0068] After PCR amplification, the target gene was recovered by gel, EcoR I single-digested, and then the digested fragment was recove...
Embodiment 2
[0073] Transformation and Screening of Multi-copy Recombinants of HBc Yeast
[0074] Extraction and linearization of multi-copy recombinant plasmids: Take 20 μg of the correctly identified four-copy plasmid and digest it with Sal I. The digested product is recovered through a nucleic acid column, and precipitated with 1 / 10 volume of 3M NaAc and 2 times the volume of absolute ethanol. Wash with 70% ethanol and redissolve in 10 μl sterile water.
[0075] Yeast Competent Preparation: GS115 strain YPD plate was streaked, and when the single clone grew out, the single clone was picked in 50mLYPD medium, cultivated overnight, and the OD 600 When it reaches 1.2-1.6, centrifuge at 1500g 4°C for 5min, wash twice with pre-cooled sterile water, wash once with pre-cooled 1M sorbitol, resuspend in 1mL pre-cooled 1M sorbitol, take 100μl competent cells and add Transfer to the above-mentioned linearized plasmid, transfer to the electroporation cup, ice-bath for 5 minutes, transform by elect...
Embodiment 3
[0077] Induced expression of HBc yeast
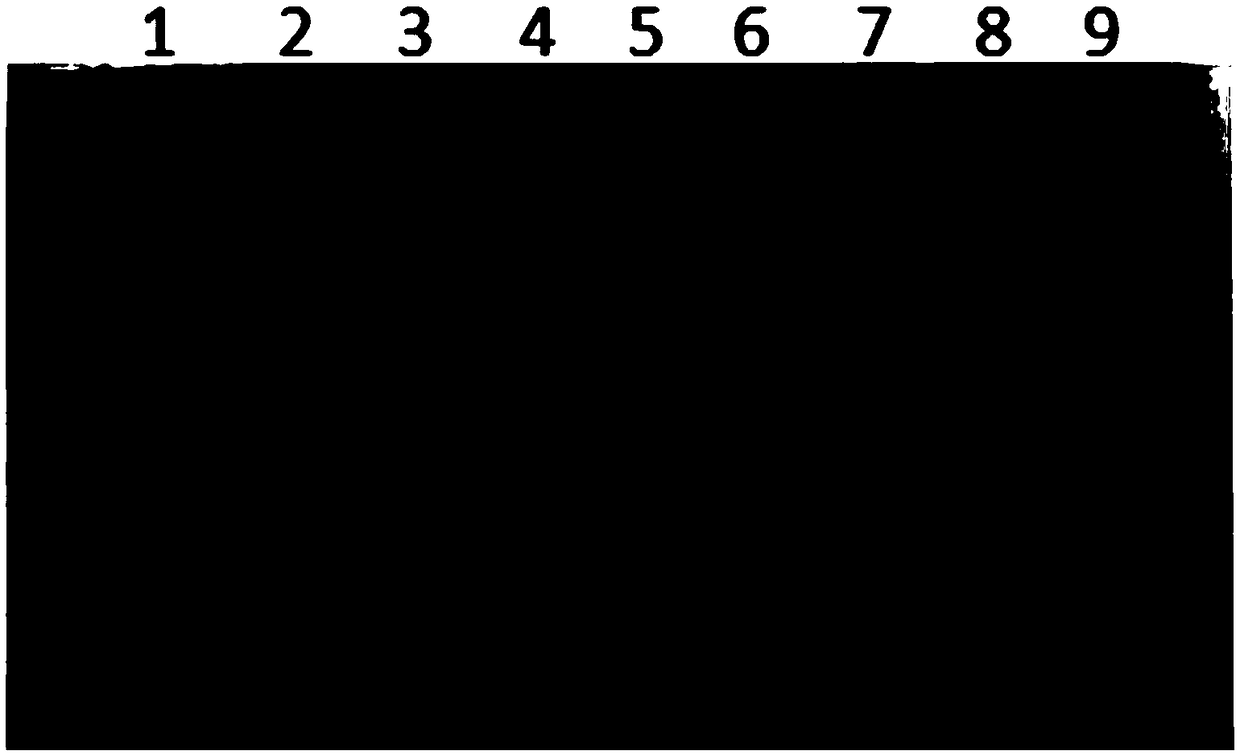
[0078] For the correctly identified transformants, take part of the strains and inoculate them in BMGY medium at 30°C for 16-18 hours, then replace them with BMMY medium to make the OD 600 =0.8-1, supplemented with 0.5% methanol every 12 hours, and continuously induced for 4 days. After the expression is finished, take 20 μL of the bacterial liquid for SDS-PAGE identification, and the expression identification results are shown in figure 2 . Compared with the control, the multi-copy HBc recombinant yeast had obvious expression band at 22kD.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com