Biological marker for detecting colorectal cancer and application thereof
A biomarker and colorectal cancer technology, applied in the field of diagnostic markers, can solve problems such as low sensitivity and specificity, poor patient compliance, invasiveness, and high cost, and achieve high specificity, simple operation, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Online big data analysis
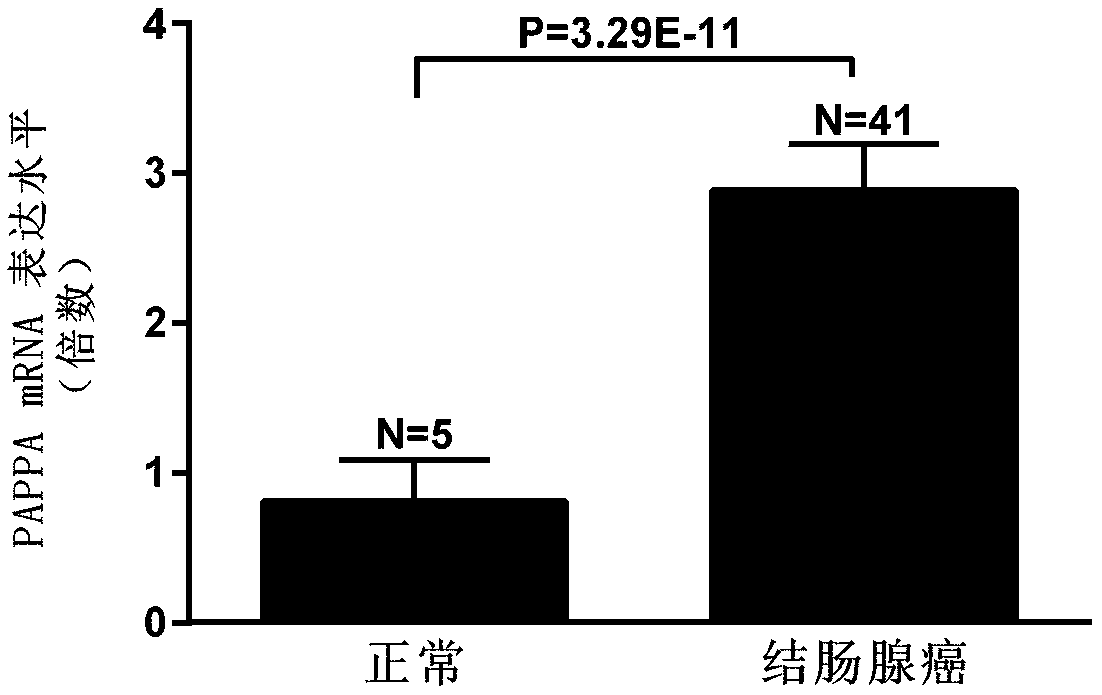
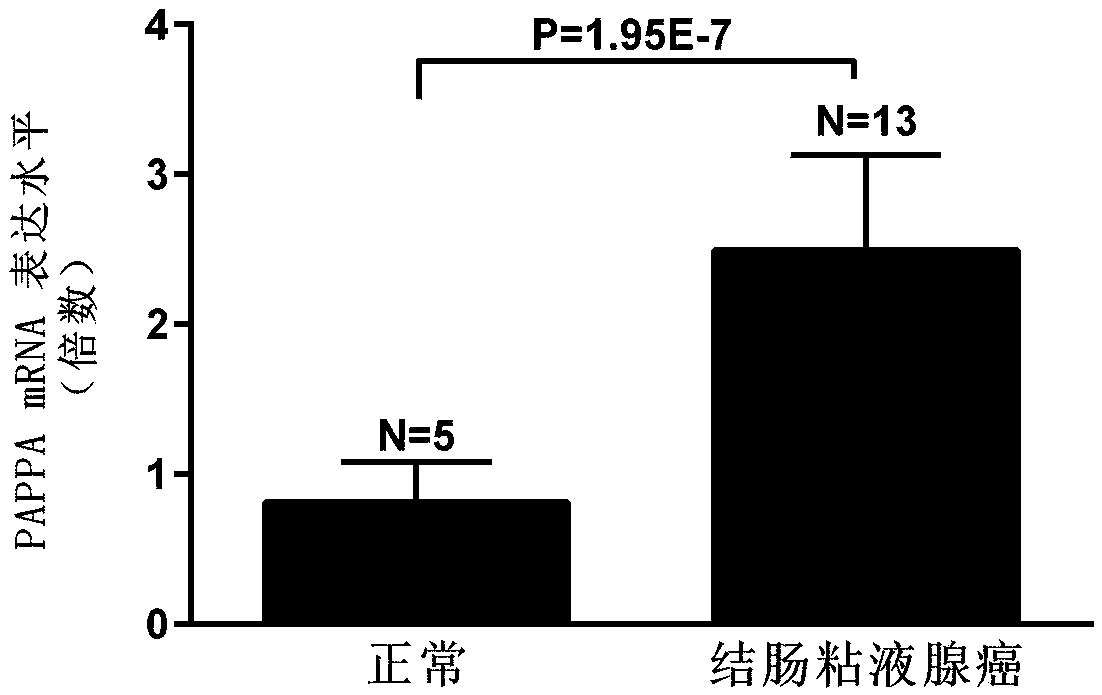
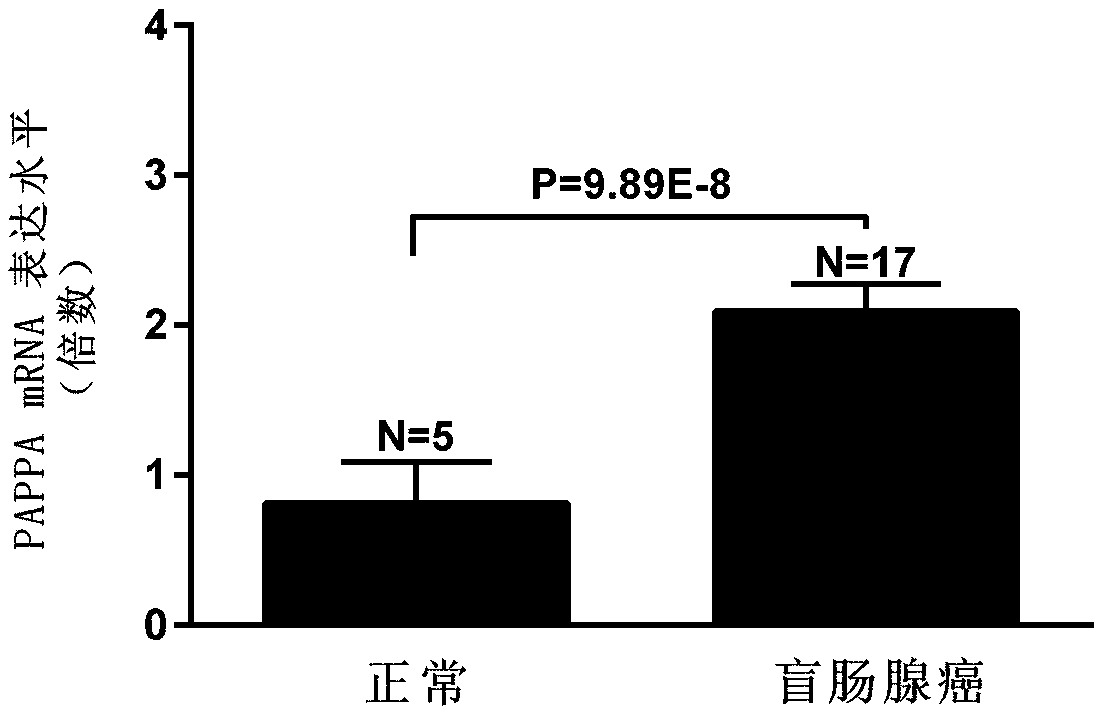
[0035] Using online gene analysis data (www.oncomine.org), PAPPA expression was significantly increased in colon adenocarcinoma compared with normal tissue (p=3.29E-11, figure 1 ), the expression was significantly increased in colonic mucinous adenocarcinoma (p=1.95E-7, figure 2 ), the expression was significantly increased in cecum adenocarcinoma (p=9.89E-8, image 3 ), the expression was significantly increased in sigmoid colorectal cancer (p=1.75E-6, Figure 4 ) was significantly increased in rectal adenocarcinoma (p=1.60E-5).
Embodiment 2
[0036] Example 2 The mRNA expression level of PAPPA by qRT-PCR
[0037] 1. Collection of human colon cancer and colitis tissue samples
[0038] Twenty-eight pairs of colorectal cancer tissue specimens and their matched normal tissue specimens, 10 pairs of colitis tissue specimens and their matched normal tissue specimens were collected from the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Jining Medical College, and then the specimens were stored at -80°C.
[0039] 2. RNA extraction
[0040] RNA was extracted from frozen colon cancer and adjacent normal colon tissues using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manual.
[0041] 3. Detection of PAPPA mRNA expression level by qRT-PCR
[0042] Use qRT-PCR to detect the mRNA expression level of PAPPA, reaction system: forward primer (20 μm) 0.15 μl, reverse primer (20 μm) 0.15 μl, cDNA 0.7 μl, H 2 O 9 μl, fluorescent dye SYBR Green (2×) 10 μl; reaction conditions: 50°C for 2 min...
Embodiment 3
[0052] Example 3 Using enzyme-linked immunosorbent assay (ELISA) to detect PAPPA in serum
[0053] 1. Collection of human serum samples
[0054] In this study, a total of 40 serum samples were obtained from Xinxiang Central Hospital, including 20 colon cancer serum samples and 20 normal human serum samples. All colorectal cancer patients were confirmed by pathological examination. All subjects were fasted for more than 8 hours before blood drawing, and about 5ml of fasting venous blood was collected, left at room temperature for 30 minutes, and then centrifuged at 3000r / min for 10 minutes to collect serum, which was frozen at -20°C for PAPPA concentration monitoring.
[0055] 2. Use enzyme-linked immunosorbent assay (ELISA) to detect the concentration of PAPPA in serum
[0056] Enzyme-linked immunosorbent assay (ELISA) was used to detect the concentration of PAPPA in serum samples. Three repetitions were set for each group of serum samples, and the OD value was detected by a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com