Light-excited formaldehyde gas sensor and preparation process thereof
A formaldehyde gas and preparation technology, applied in the field of sensors, can solve the problems of detonating flammable gases, shortening service life, increasing energy consumption, etc., and achieve the effect of strengthening gas-sensing selectivity, improving adsorption capacity, and increasing surface electron concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
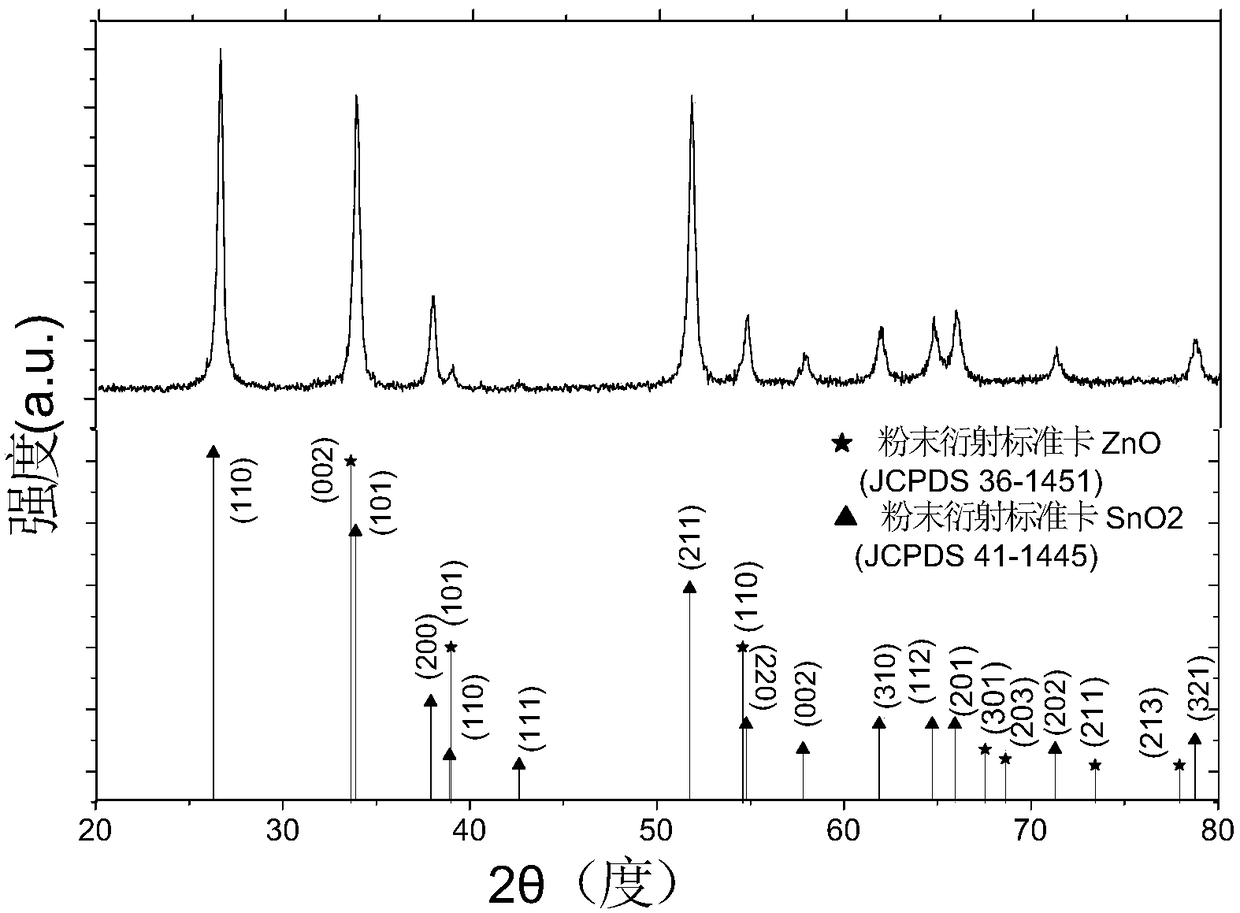
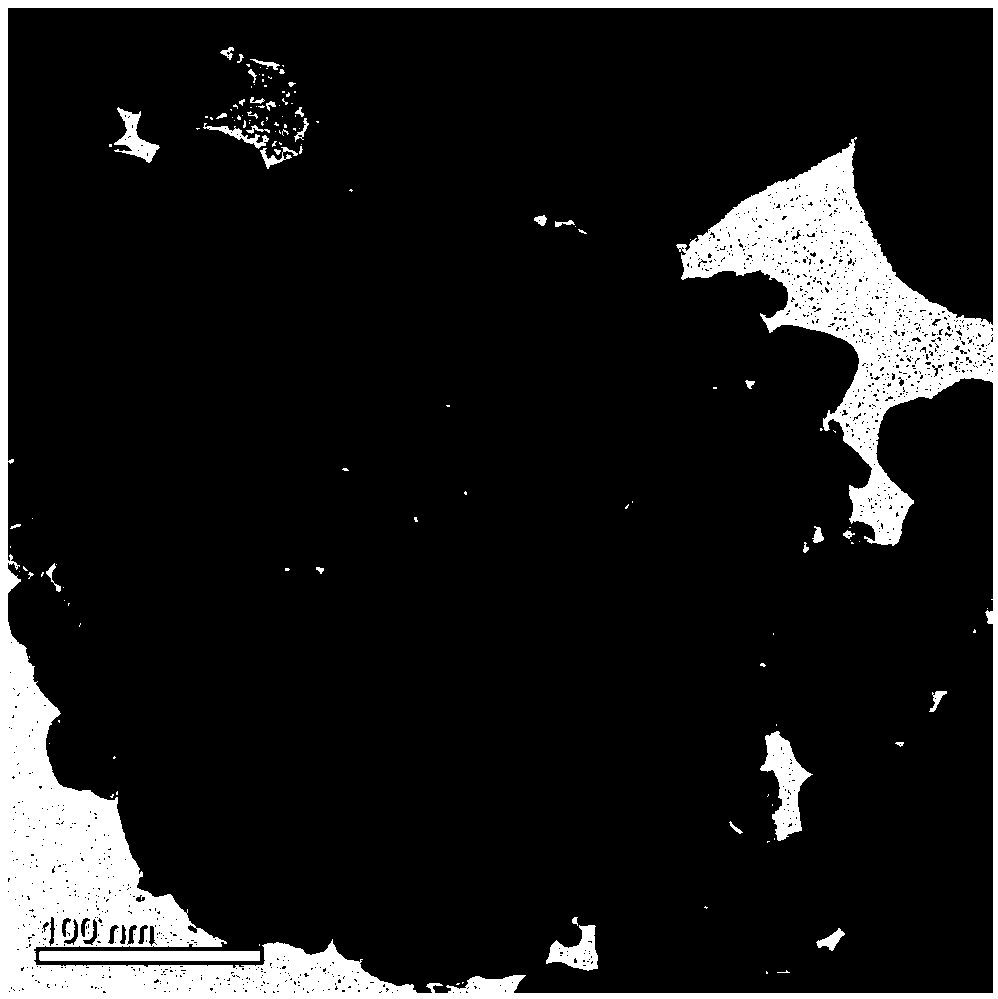
[0031] A light-excited formaldehyde gas sensor, including a light source, a gas-sensitive material and a substrate, the gas-sensitive material is evenly coated on the surface of the substrate, and the gas-sensitive material is composed of a hollow microsphere zinc oxide tin dioxide double-layer heterojunction Composite nanomaterials, coated with a thickness of 1 μm to 100 μm. The hollow microsphere zinc oxide tin dioxide double-layer heterojunction composite nanomaterial is composed of a tin dioxide nano hollow sphere in the inner layer and a zinc oxide coating shell in the outer layer, and the zinc oxide coating shell accounts for 100% of the composite nanomaterial. 10%-30% of the mass. The diameter of the composite nanomaterial sphere is 100nm-300nm, the diameter of the inner tin dioxide hollow nanosphere is about 100nm-250nm, the thickness of the tin dioxide spherical shell is about 10nm-20nm, and the outer zinc oxide shell is about 20nm -40nm, the gap between the two laye...
Embodiment 2
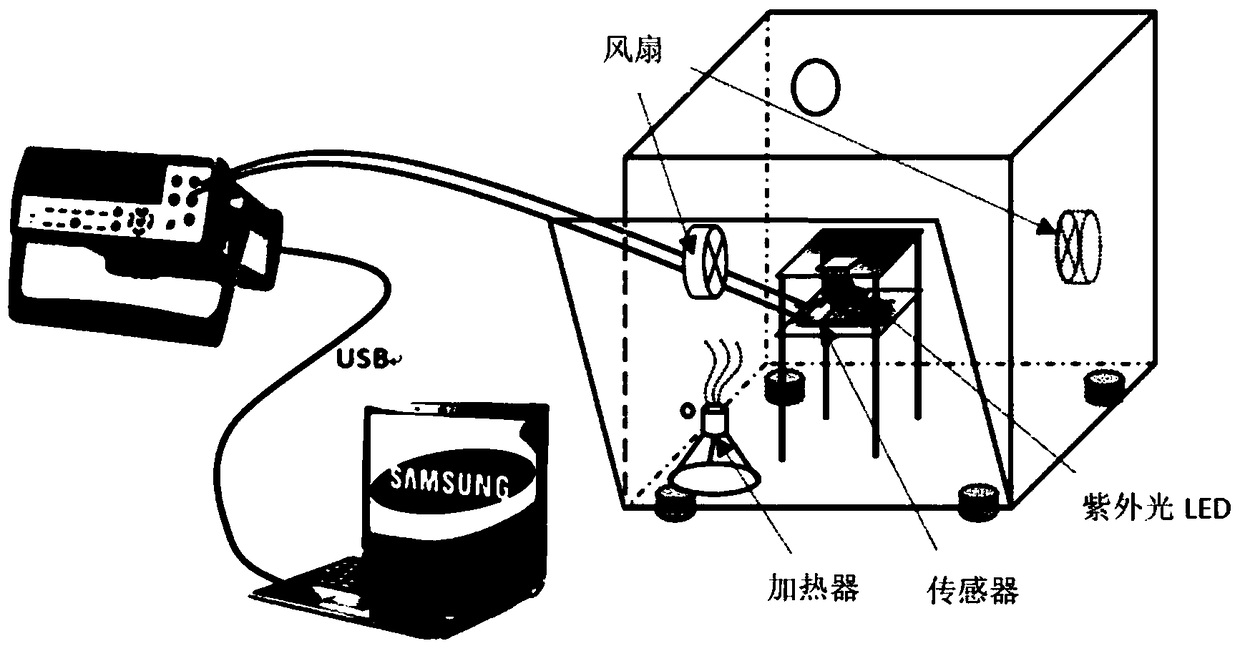
[0039] see image 3 As shown, a light-excited formaldehyde gas sensor testing system provided in this embodiment includes a multimeter, a fan, a sensor, an ultraviolet LED, and a heater. The gas sensor is a thick film resistive sensor, and the thickness of the sensitive material is about 1 μm to 100 μm.
[0040] Wherein, the gas sensor includes a semiconductor gas sensitive material and a substrate.
[0041] The semiconductor gas-sensing material is a hollow microsphere zinc oxide tin dioxide double-layer heterojunction composite nanomaterial, which is prepared by template method and high temperature calcination. The diameter of a single hollow nanosphere is about 100-300nm, and the thickness of the spherical shell is 30-50nm. An organic solvent is applied to the surface of the substrate.
[0042] The substrate is a Si substrate with Au electrodes.
[0043] Among them, the ultraviolet light LED is a bead-type LED light source with a light wavelength of 365nm and a power of ...
Embodiment 3
[0049] A light-excited formaldehyde gas sensor, including a light source, a gas-sensitive material and a substrate, the gas-sensitive material is evenly coated on the surface of the substrate, and the gas-sensitive material is composed of a hollow microsphere zinc oxide tin dioxide double-layer heterojunction Composite nanomaterials, coated with a thickness of 1 μm to 100 μm. The hollow microsphere zinc oxide tin dioxide double-layer heterojunction composite nanomaterial is composed of a tin dioxide nano hollow sphere in the inner layer and a zinc oxide coating shell in the outer layer, and the zinc oxide coating shell accounts for 100% of the composite nanomaterial. 10%-30% of the mass. The diameter of the composite nanomaterial sphere is 100nm-300nm, the diameter of the inner tin dioxide hollow nanosphere is about 100nm-250nm, the thickness of the tin dioxide spherical shell is about 10nm-20nm, and the outer zinc oxide shell is about 20nm -40nm, the gap between the two laye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com