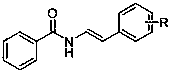
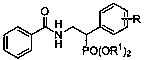
Beta-aminophosphonic acid derivative and preparation method thereof
An aminophosphonic acid and derivative technology, which is applied in the field of preparation of organic compounds, can solve the problems of harsh reaction conditions, difficult to obtain raw materials, and many reaction steps, and achieves the effects of mild reaction conditions, few steps, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of 2-amino-1-phenylethylphosphonic acid
[0045] With N-(styryl) benzamide as raw material, the reaction steps are as follows:
[0046] (1) Add N-(styryl)benzamide (223 mg, 1 mmol), dimethyl phosphite (220 mg, 2 mmol), manganese acetate (580 mg, 2.5 mmol) into the reaction flask, anhydrous Potassium carbonate (276 mg, 2 mmol) and methanol (10 mL). The mixture was stirred and reacted at room temperature, followed by TLC until the reaction was completely completed (about 0.5 hours);
[0047] (2) The crude product obtained after the reaction finishes is separated by column chromatography (dichloromethane: methanol=100:1) to obtain (2-benzamido-1-styryl) dimethylphosphonate (281 mg, 0.85 mmol, 85% yield). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.19 – 8.06 (m, 2H), 7.64 – 7.60 (m, 2H), 7.52(dd, J = 15.0, 7.5 Hz, 3H), 7.45 – 7.37 (m, 5H), 3.78 (s, 3H), 3.75 (s, 3H).
[0048] (3) The above product (2-benz...
Embodiment 2
[0051] Example 2: Synthesis of 2-amino-1-(2-tolyl)ethylphosphonic acid
[0052] With N-(2-methylstyryl) benzamide as raw material, the reaction steps are as follows:
[0053] (1) Add N-(2-methylstyryl)benzamide (237 mg, 1 mmol), dimethyl phosphite (220 mg, 2 mmol), manganese acetate (580 mg, 2.5 mmol), anhydrous potassium carbonate (276 mg, 2 mmol) and methanol (10 mL). The mixture was stirred and reacted at room temperature, followed by TLC until the reaction was completely completed (about 0.5 hours);
[0054] (2) The crude product obtained after the reaction is separated by column chromatography (dichloromethane:methanol=100:1) to obtain (2-benzamido-1-(2-tolyl)vinyl)dimethyl Phosphonate (280 mg, 0.81 mmol, 81% yield). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.19–8.06 (m, 2H), 7.70–7.50 (m, 3H), 7.40–6.80 (m, 4H), 3.78 (s, 3H), 3.75 (s, 3H) , 1.75 (s, 3H);
[0055] (3) Add the above product (280 mg, 0.81 mmol), Raney-Ni (56.4 m...
Embodiment 3
[0057] Example 3: Synthesis of 2-amino-1-(2-methoxyphenyl)ethylphosphonic acid
[0058] With N-(2-methoxy styryl) benzamide as raw material, the reaction steps are as follows:
[0059] (1) Add N-(2-methoxystyryl)benzamide (253 mg, 1 mmol), dimethyl phosphite (220 mg, 2 mmol), manganese acetate (580 mg, 2.5 mmol), anhydrous potassium carbonate (276 mg, 2 mmol) and methanol (10 mL). The mixture was stirred and reacted at room temperature, followed by TLC until the reaction was completely completed (about 0.5 hours);
[0060] (2) The crude product obtained after the reaction is separated by column chromatography (dichloromethane:methanol=100:1) to obtain (2-benzamido-1-(2-methoxyphenyl)vinyl) Dimethylphosphonate (289 mg, 0.80 mmol, 80% yield). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.21–8.01 (m, 2H), 7.70–7.50 (m, 4H), 7.40–6.80 (m, 5H), 3.79 (s, 3H), 3.77 (s, 3H), 3.74 (s, 3H);
[0061] (3) Add the above product (289 mg, 0.80 mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com