Tabletted Moringa tree leaf extract candy, and production method and application thereof
A technology for compressing candy and Moringa leaves, which can be used in food ingredients, applications, and confectionery containing natural extracts. It can solve the problems of anti-oxidative enzyme system homeostasis and aggravate vascular endothelial inflammation damage, and achieve stable quality. Controlling, complete absorption, unique taste and flavor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 Moringa leaf extract compressed tablet candy
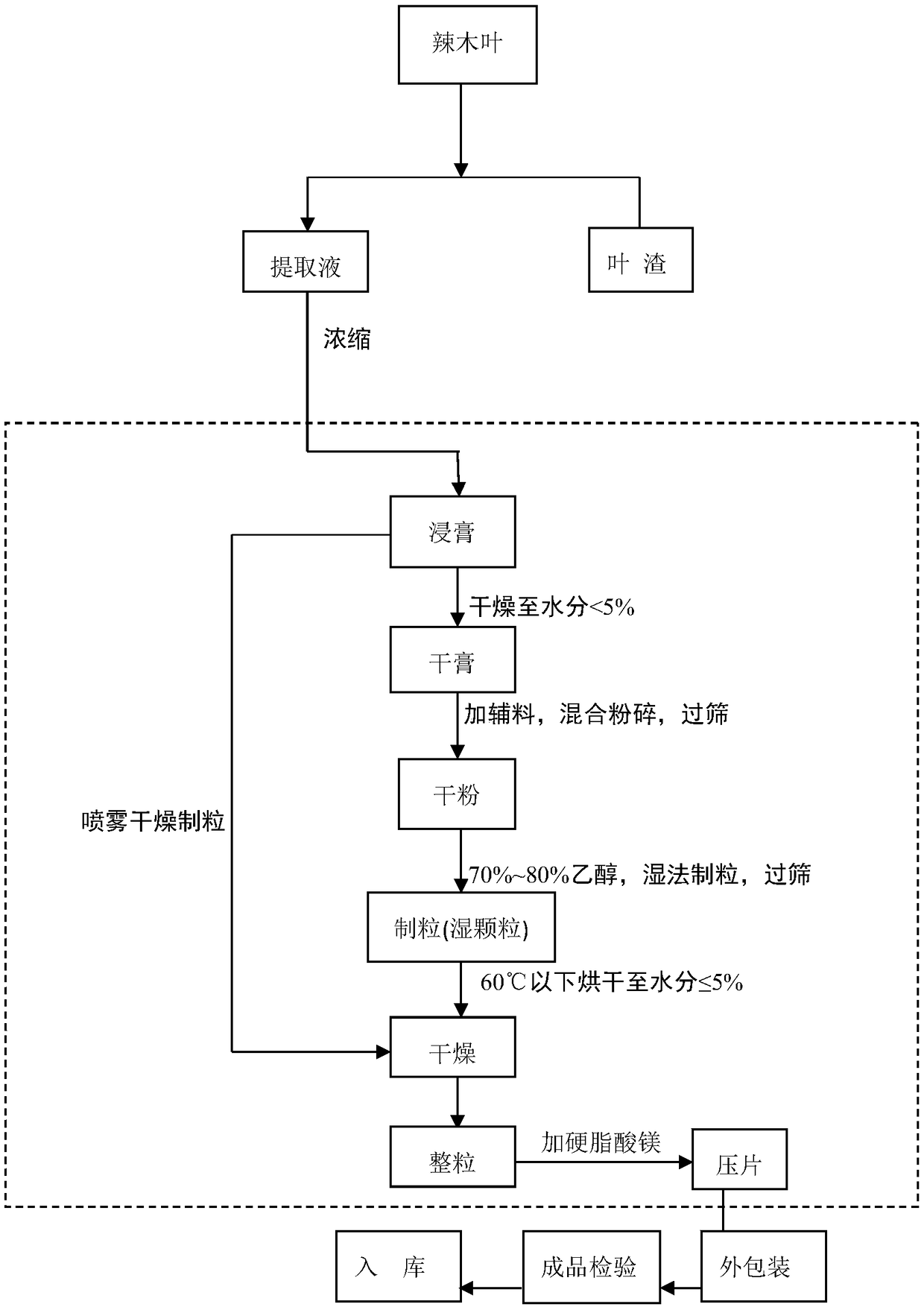
[0032] figure 1 Process flow diagram for the production of tableted candy from Moringa oleifera leaf extract. The specific preparation process will be described below in conjunction with the flowchart.
[0033] 1. Extraction and purification: Take the dried Moringa leaves that have passed the inspection, weigh them, put them into the multifunctional extraction tank through the feeding port, and feed them to the extraction tank at a material-liquid ratio (mass and volume ratio) of 1kg: (6-15) L Add purified water, distilled water or qualified drinking water, heat and reflux at 100°C for 2 to 3 times, each extraction for 1h (starting from boiling), filter the extract through a 100-mesh sieve, and then store it at 2-4°C (not suitable for freezing) ) to stand still for 48 hours, take the supernatant, combine the supernatants, the relative density of the extract is 1.1-1.12 is the best, and prep...
Embodiment 2
[0041] The preparation of embodiment 2 Moringa leaf extract compressed tablet candy
[0042] Different from the auxiliary materials described in the molding process of step 4 in Example 1, the auxiliary materials described in this embodiment are powdered sugar, stevioside and mannitol, and the dry extract of Moringa oleifera leaves, powdered sugar, stevioside, and mannitol are The mass ratio is 1:0.5:0.5:0.5 and the other steps are the same.
[0043] The composition of the above-mentioned Moringa leaf extract tablet candy includes: Moringa leaf dry paste: auxiliary materials (sugar powder, stevioside and mannitol): the mass ratio of magnesium stearate is 1:1.5:0.13.
Embodiment 3
[0044] The preparation of embodiment 3 Moringa leaf extract compressed tablet candy
[0045] Different from the auxiliary materials described in the molding process of step 4 in Example 1, the auxiliary materials described in this embodiment are powdered sugar, stevioside, mannitol and lactose, and the powdered sugar, stevioside, and mannitol are in a mass ratio of 1:1 : Mix the amount of 1, and the other steps are the same.
[0046] The composition of the above-mentioned Moringa leaf extract tablet candy includes: Moringa leaf dry paste: auxiliary materials (sugar powder, stevioside and mannitol): the mass ratio of magnesium stearate is 35:60:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com