Pure organic dyes based on bis(thienopyrrole) benzothiadiazole π bridges and their applications in dye-sensitized solar cells
A technology of benzothiadiazole and solar cells, which is applied in photosensitive pure organic dyes, dye-sensitized solar cells, and dye-sensitized solar cells, and can solve the problems of cumbersome synthesis steps, high price, and difficult separation and purification. , to achieve the effect of simple synthesis method, cheap raw materials and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
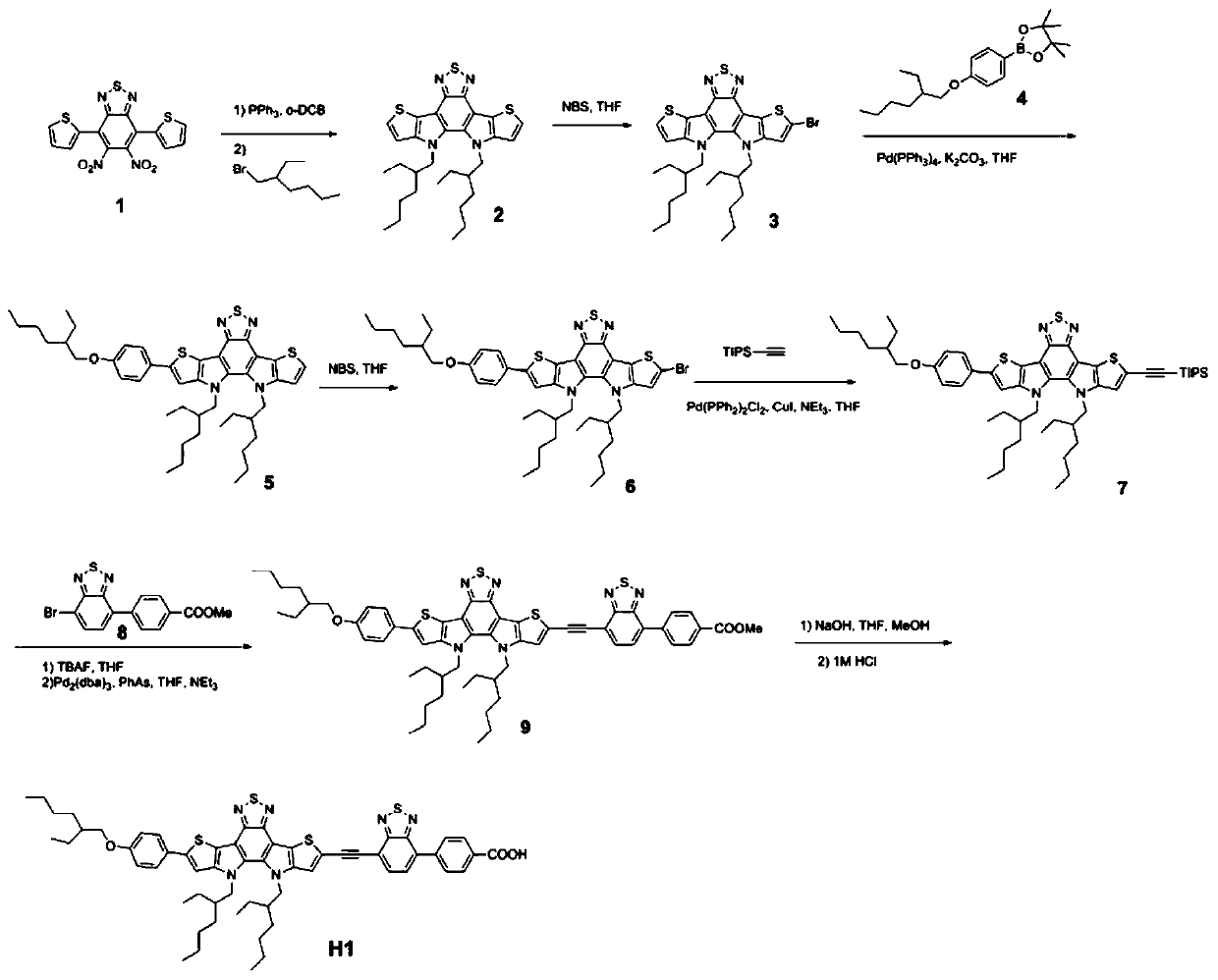
[0049] Synthesis of Pure Organic Dye H1 Based on Bis(thienopyrrole)benzothiadiazole π Bridge
[0050] (1) Synthesis of Compound 2
[0051]
[0052] Under the protection of argon, 1.25 g (3.20 mmol) of compound 1 was added to a 100 mL two-neck round bottom flask with 20 mL of o-DCB as the solvent, and the reaction system was heated to 180 ° C for 16 h. After cooling to room temperature, the reaction solvent was distilled off under reduced pressure, and the residue was separated and purified by silica gel column chromatography. Petroleum ether: ethyl acetate (2:1) was used as the mobile phase, and the obtained yellow solid product was directly put into the next reaction. Take a 100mL two-neck round bottom flask, add the yellow solid prepared above, 3.71g (19.20mmol) bromoisoctane, 2.43g (32mmol) KOH, 100mg (0.62mmol) KI and 30mL DMSO solvent. The reaction was stirred at 85° C. for 12 h under the protection of argon. After the reaction solution was cooled to room temperatur...
Embodiment 2
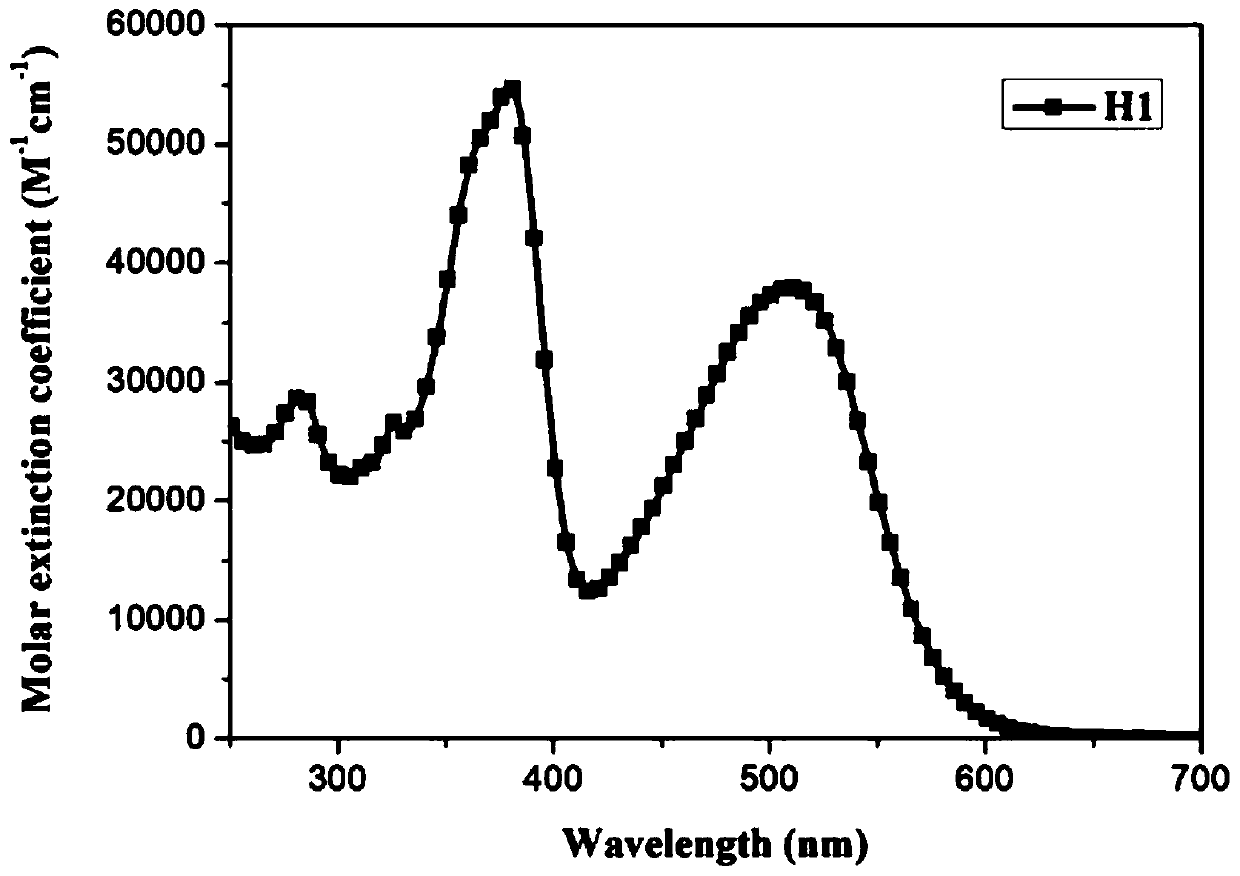
[0072] To the ultraviolet-visible absorption spectrum test of embodiment 1, ultraviolet-visible absorption spectrum is as figure 2 shown.
[0073] Solvent: dichloromethane
[0074] Concentration: 2×10 -5 m
[0075] Temperature: room temperature
[0076] Instrument: Shimadzu UV-2450 UV-Vis spectrophotometer
[0077] The resulting data are summarized in Table 1
[0078] The ultraviolet-visible spectral data of table 1 embodiment 1 dyestuff
[0079]
Embodiment 3
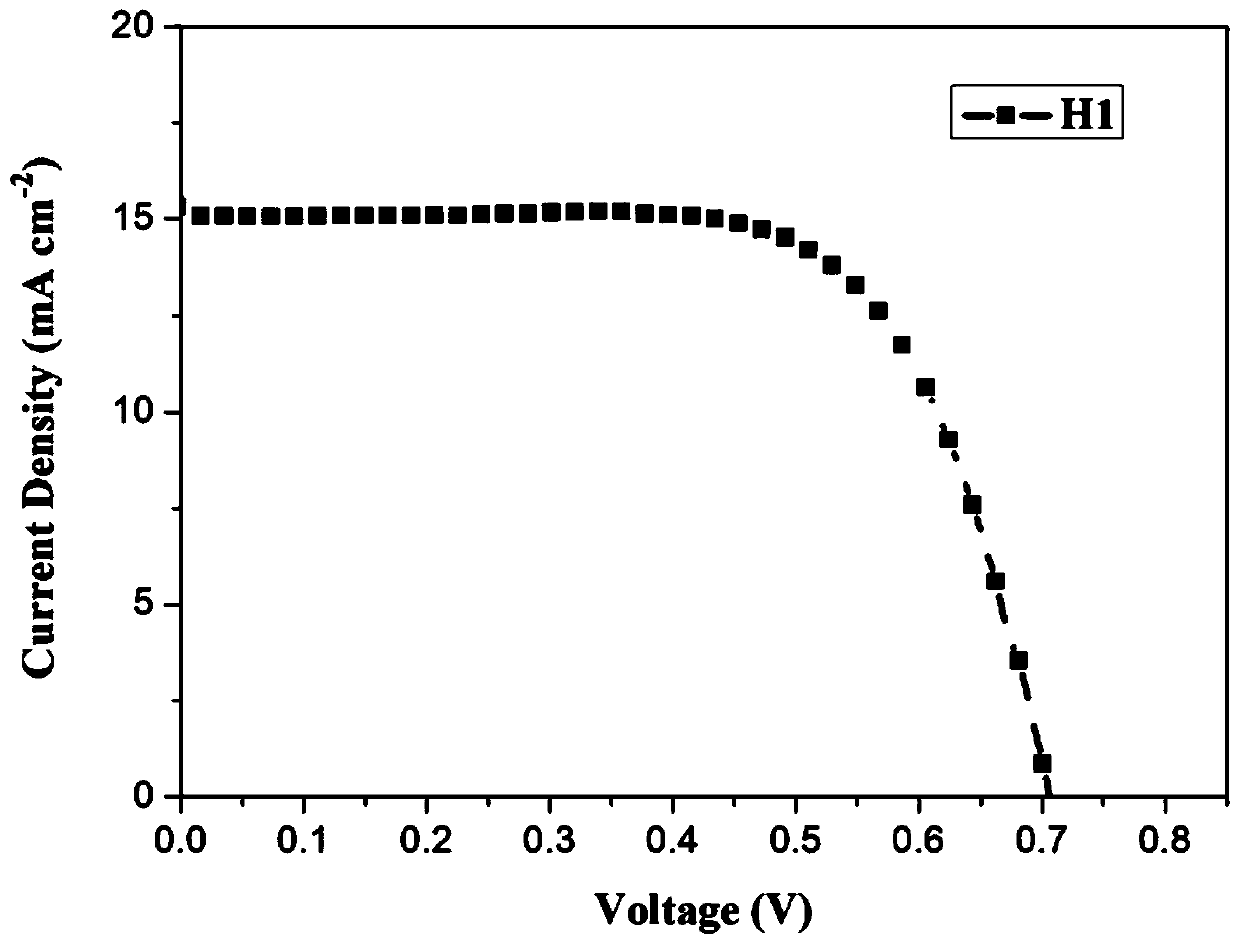
[0081] The making of dye-sensitized solar cell among the present invention is as follows:
[0082] a. Pretreatment of conductive glass (FTO): Fully clean the conductive glass in degreasing agent, absolute ethanol and deionized water with ultrasonic waves, and then dry it for later use;
[0083] b. TiO 2 Preparation of nanocrystalline particles and their slurry: at room temperature, 10mL Ti(OBu) 4 After stirring the mixture with 20mL of EtOH for 10 minutes, add 18mL of acetic acid and 50mL of deionized water to the above solution under vigorous stirring and keep stirring for 1h, then transfer the mixture into an autoclave at 230°C for 12h, and naturally cool to room temperature , filter the resulting suspension, wash with deionized water and ethanol several times respectively, and dry in an oven at 50°C for 6h to dry to obtain TiO with a particle size of about 20nm. 2 Nanocrystalline particles;
[0084] c. Take TiO 2 Add 1.0g of nanocrystalline particles to 8.0mL of ethanol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com