Cross-linked dextran microparticles for hemostasis, and preparation method of cross-linked dextran microparticles
A technology of cross-linked dextran and dextran, which is applied in the fields of applications, pharmaceutical formulations, and medical science, and can solve problems such as difficult biodegradation, undisclosed preparation methods of cross-linked dextran, and inapplicability for hemostasis. The preparation method is simple and easy to operate, non-immunogenic, and the effect of lowering the price is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of cross-linked dextran microparticles of this embodiment includes the following steps:
[0044] ① Add 300 mL of acetone to a 500 mL three-necked flask, and add 20 g of dextran (also known as dextran, which is synthesized from sucrose through Leuconostoc mesenteroides) fermentation at room temperature (15-30°C). (Molecular weight 40,000) dry powder, stir to make the dry powder evenly dispersed in acetone.
[0045] ②The 0.2mol / L sodium hydroxide aqueous solution is placed in the dropping funnel, and 120mL of sodium hydroxide solution is added dropwise to the three-necked flask of step ①, and the dripping is completed within 30 minutes; while stirring, the flocculation in the three-necked flask The material is evenly dispersed.
[0046] During the dropwise addition of the sodium hydroxide solution, the three-necked flask was gradually increased in temperature, increasing by 4 to 6°C (5°C in this embodiment) every 10 min, raising the temperature to 40°C an...
Embodiment 2
[0055] The preparation method of the cross-linked dextran microparticles of this embodiment is the same as that of embodiment 1, except that:
[0056] Step ② The temperature of the material in the bottle is gradually increased to 45°C.
[0057] Step ③ After adding epichlorohydrin, the ratio of the amount of dextran to epichlorohydrin is 5:4. After the addition of epichlorohydrin, the reaction was continued with stirring at 45°C for 8h.
[0058] The photomicrograph of the cross-linked dextran microparticles (sieved) prepared in this example is shown Figure 5 .
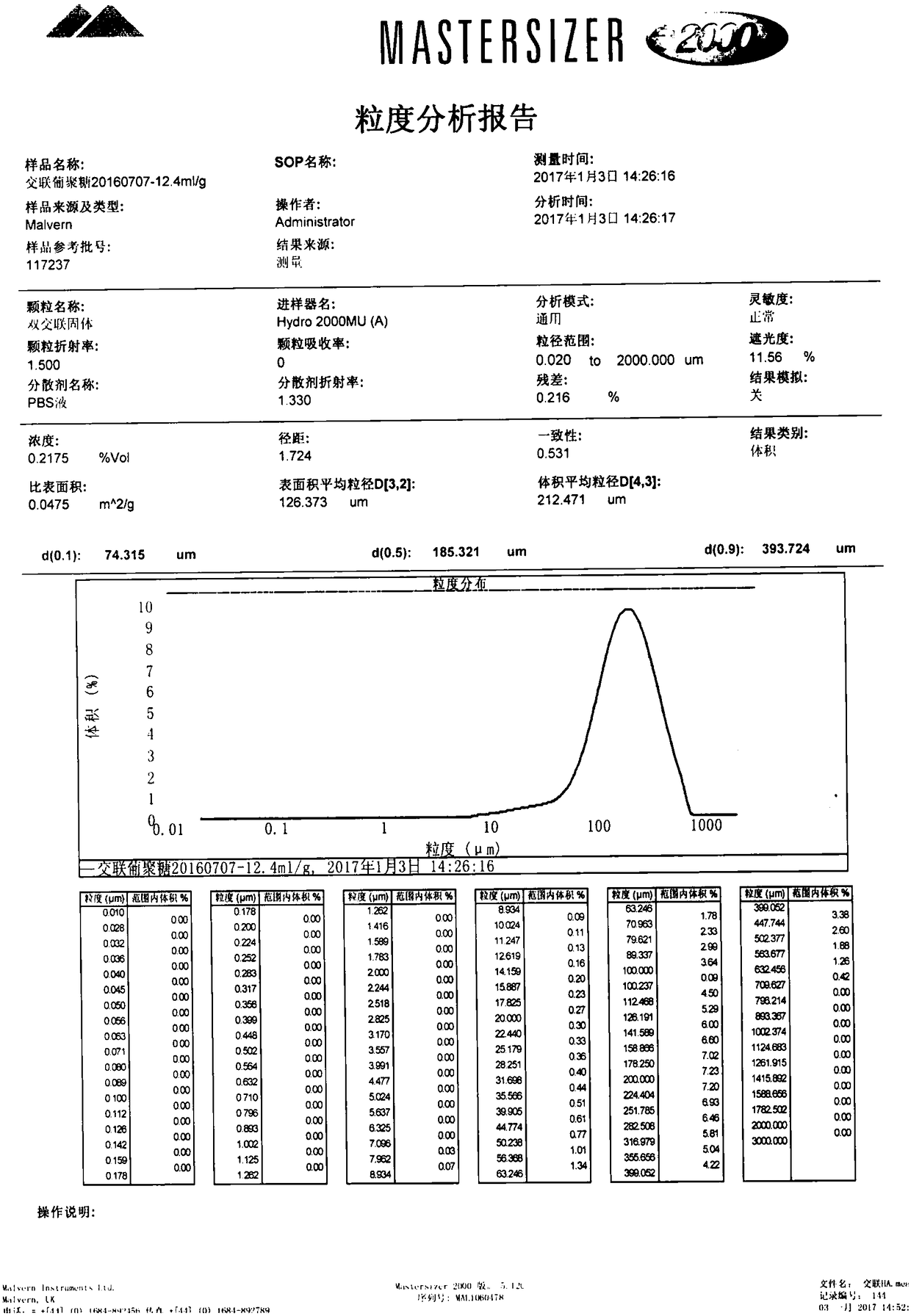
[0059] The particle size distribution diagram of the cross-linked dextran microparticles prepared in this example before sieving is shown in Image 6 The average particle size of the prepared particles is 134.922μm.
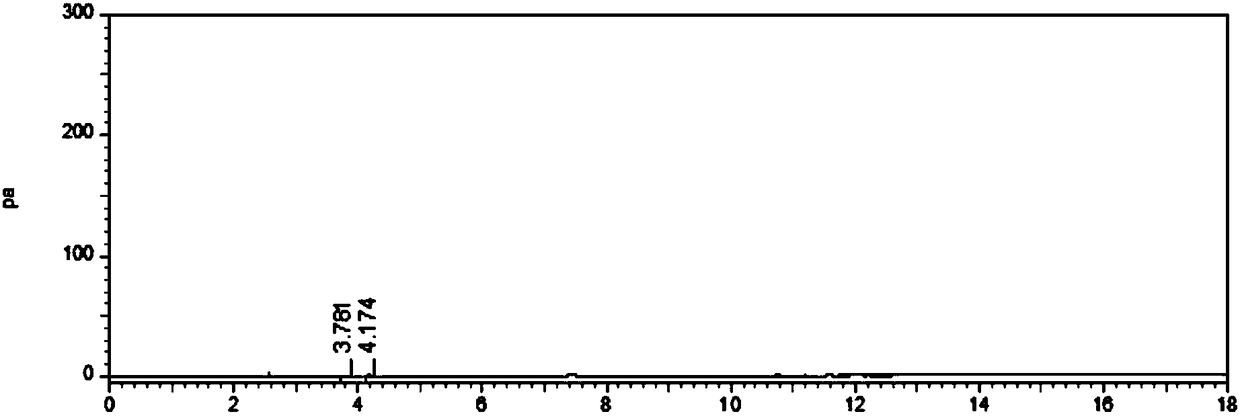
[0060] Detect the residues of solvent acetone and crosslinker epichlorohydrin in the dried microspheres obtained in step ⑥ by gas chromatography, Figure 7 The gas chromatogram of the cross-linked dextran micropar...
Embodiment 3
[0062] The preparation method of the cross-linked dextran microparticles of this embodiment is the same as that of embodiment 1, except that:
[0063] Step ② The temperature of the material in the bottle is gradually increased to 35°C.
[0064] Step ③ After adding epichlorohydrin, the ratio of the amount of dextran to epichlorohydrin is 5:5. After the addition of epichlorohydrin, continue to stir and react at 35°C for 15h.
[0065] The photomicrograph of the cross-linked dextran microparticles (sieved) prepared in this example is shown Picture 9 .
[0066] The particle size distribution diagram of the cross-linked dextran microparticles prepared in this example before sieving is shown in Picture 10 , The average particle size of the prepared particles is 348.129μm.
[0067] Detect the residues of solvent acetone and crosslinker epichlorohydrin in the dried microspheres obtained in step ⑥ by gas chromatography, Picture 11 The gas chromatogram of the cross-linked dextran microparticle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com