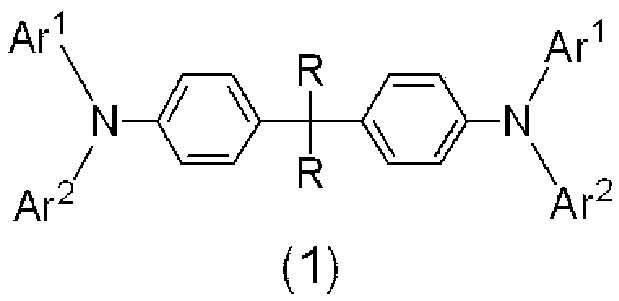
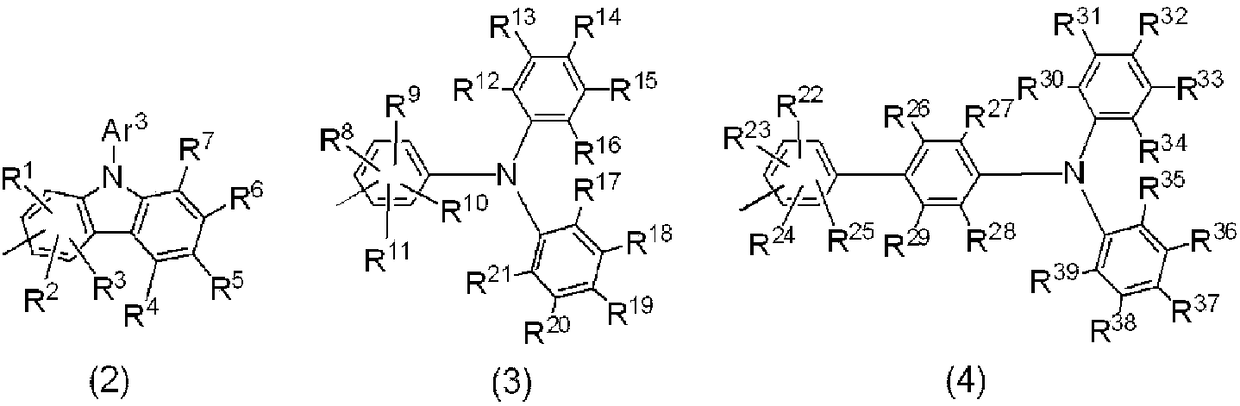
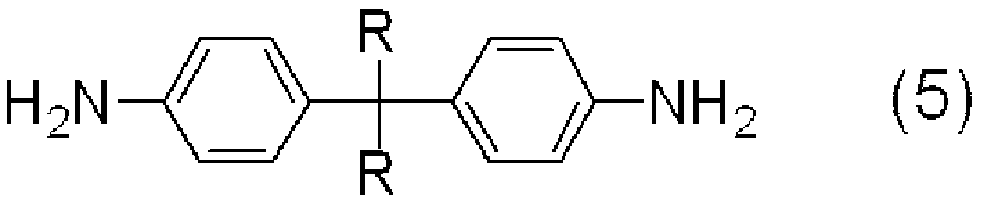
Arylamine derivative and use thereof
A derivative, arylamine technology, applied in the field of arylamine derivatives to achieve the effect of high charge transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0180] As the organic solvent used in the preparation of the varnish for forming a hole injection layer, a highly soluble solvent capable of favorably dissolving the above-mentioned hole injection material and, if necessary, an electron-accepting dopant substance can be used. The high solubility solvent can be used individually by 1 type or in mixture of 2 or more types, and the usage-amount can be made into 5-100 mass % with respect to the whole solvent used for a varnish.
[0181] Examples of such highly soluble solvents include N-methylformamide, N,N-dimethylformamide, N,N-diethylformamide, N-methylacetamide, N,N- Dimethylacetamide, N-methylpyrrolidone, 1,3-dimethyl-2-imidazolinone, etc.
[0182] It is preferable that both the charge-transporting substance and the electron-accepting dopant substance are completely dissolved in the above-mentioned organic solvent, or become a state of being uniformly dispersed. If it is considered that the space provided to the organic EL el...
Embodiment 1-1
[0220] [Example 1-1] Synthesis of Arylamine Derivatives 1
[0221] (1) The first process
[0222] [chemical 20]
[0223]
[0224] Put 5.77 g of 4-bromostyrene, 5.01 g of 4,4'-(hexafluoroisopropylidene) diphenylamine, and Pd(dba) into the flask 2After adding 0.174 g and 4.04 g of sodium tert-butoxide, the inside of the flask was replaced with nitrogen. Next, 75 mL of toluene and 2.0 mL (concentration: 67 g / L) of a toluene solution of di-tert-butyl(phenyl)phosphine prepared beforehand were added, and stirred at 80° C. for 1 hour. After the stirring was completed, the reaction mixture was cooled to room temperature, ethyl acetate and ion-exchanged water were mixed, and liquid separation was performed. The obtained organic layer was washed sequentially with ion-exchanged water and saturated brine, and dried over magnesium sulfate. It was filtered, and the solvent was distilled off under reduced pressure, then separated and purified by silica gel column chromatography (devel...
Embodiment 1-2
[0232] [Example 1-2] Synthesis of Arylamine Derivatives 2
[0233] [chem 22]
[0234]
[0235] Put 1.46g of the intermediate 1, 4-bromo-N,N-diphenylaniline 1.62g and Pd(dba) obtained in the first step above into the flask. 2 After adding 0.146 g and 0.676 g of sodium tert-butoxide, the inside of the flask was replaced with nitrogen. Next, 20 mL of toluene and 1.7 mL (concentration: 60 g / L) of a toluene solution of tri-tert-butylphosphine prepared in advance were added, and stirred at 80° C. for 2 hours. After the stirring was completed, the reaction mixture was cooled to room temperature, mixed with ion-exchanged water, and subjected to liquid separation treatment. The obtained organic layer was washed sequentially with ion-exchanged water and saturated brine, and dried over magnesium sulfate. This was filtered, and the solvent was distilled off under reduced pressure, followed by separation and purification by silica gel column chromatography (developing solvent: n-hexa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com