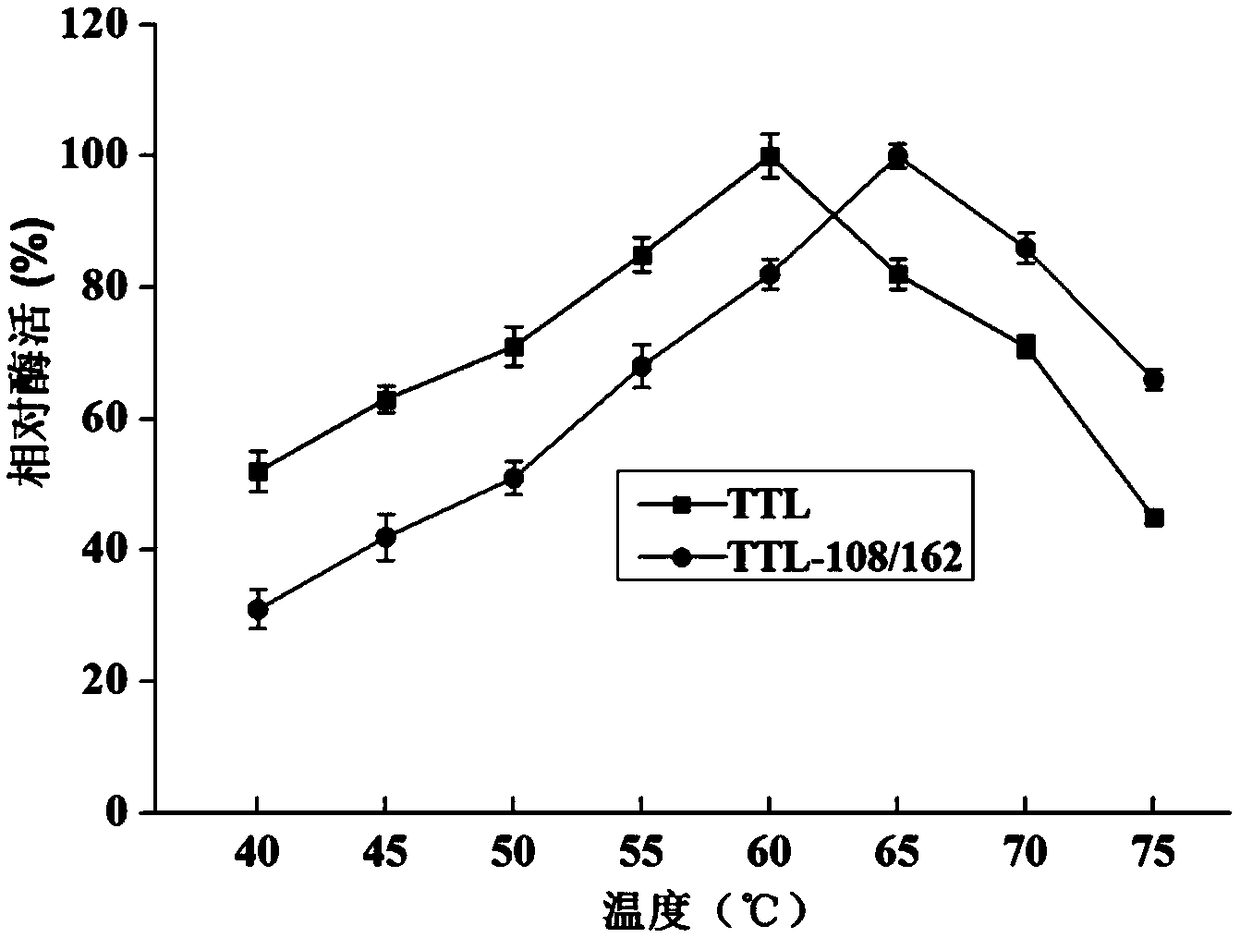
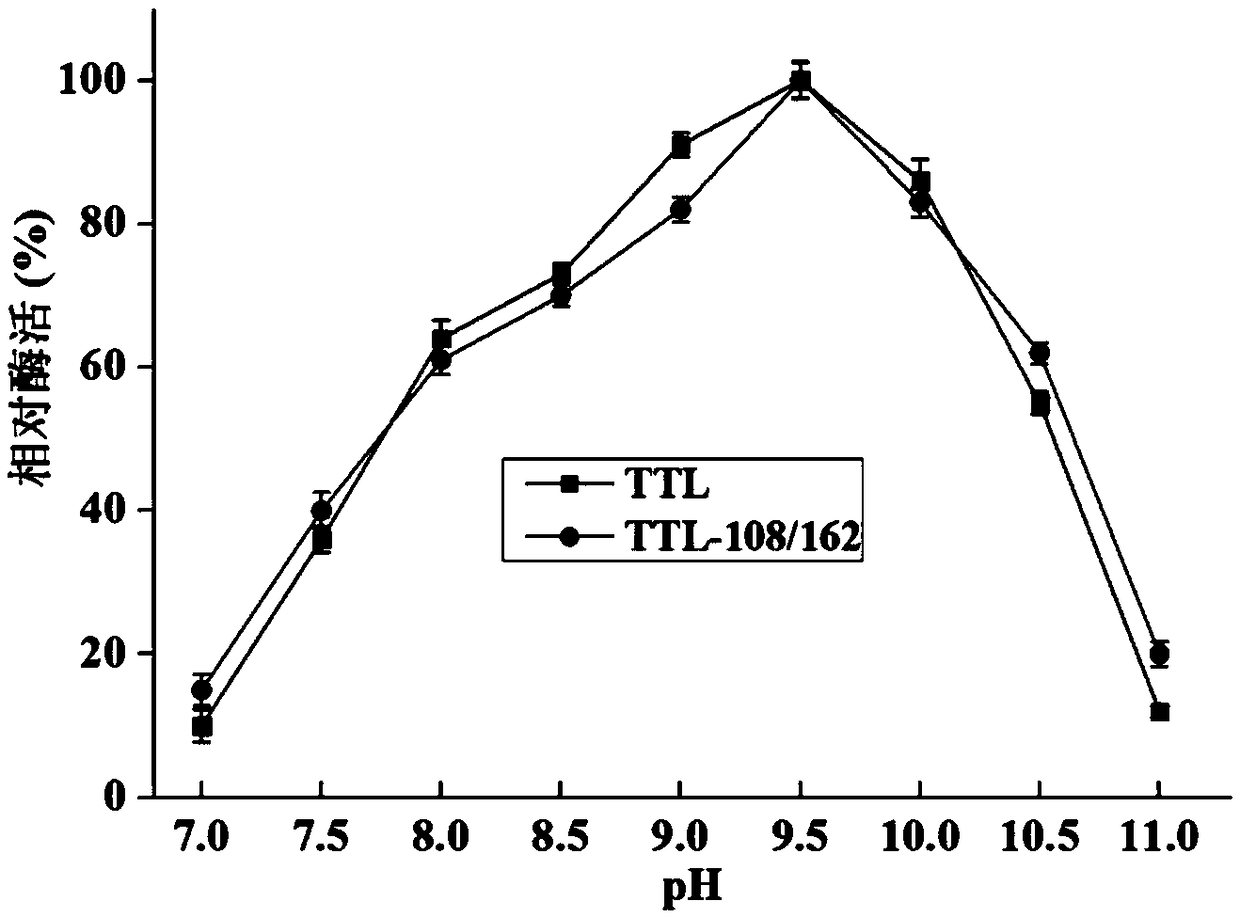
Lipase mutant with improved thermal stability and applications thereof
A technology of thermal stability and lipase, applied in the field of molecular biology, can solve the problems of lipase inactivation and denaturation, volatile inactivation, TTL instability, etc., and achieve the effect of good thermal stability and improved thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, construction of lipase TTL expression vector
[0028] Analysis of the lipase TTL gene reported on the NCBI website (Genebank: JF414585.1) revealed that the full length of the TTL lipase gene was 1083 bp, including 4 exons and 3 introns. Through comparison, it was found that the full length of its open reading frame was 876bp, encoding 291 amino acids, and the first 17 amino acids at the N-terminal of the protein were signal peptides. According to the searched TTL gene sequence, the TTL gene ttl was synthesized by the whole gene synthesis technology. A pair of primers were designed according to the sequence of the synthetic gene ttl (primer sequences were fw: 5'-agtcgaattcTCTCC AGTCAGACGTGAGGTT-3' and rev: 5'-ttctctagaTTACAAACAAGTACCAATCAA-3') to amplify the mature peptide gene tll- 1. Cloning the amplified tll-1 into the vector pPICZαA to obtain the recombinant vector pPICZαA-ttl-1.
Embodiment 2
[0029] Embodiment 2, construction disulfide bond mutant
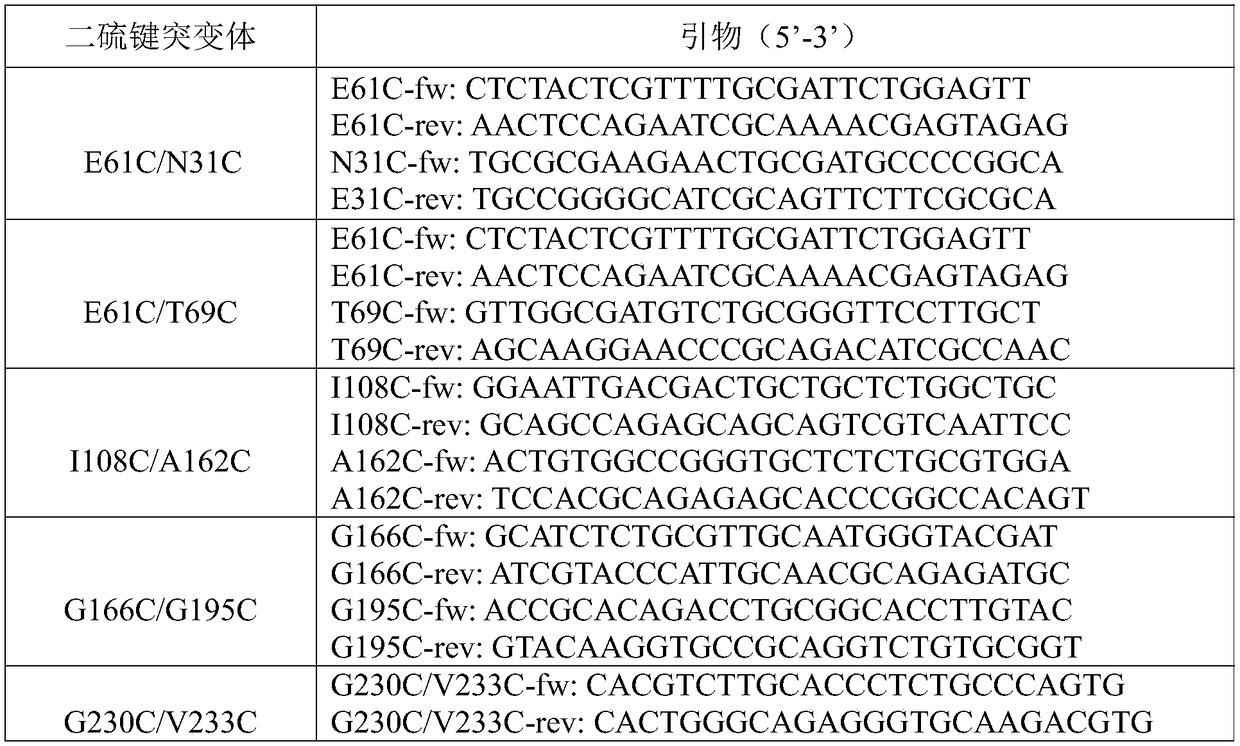
[0030] First, the three-dimensional protein structure of TTL was obtained through the homology modeling software Modeller, and then the molecular dynamics simulation of TTL was performed through the molecular dynamics software Gromacs, and the flexible region of the TTL protein was found according to the results of the molecular dynamics simulation. Add disulfide bonds in flexible regions of the protein. Five pairs of disulfide bonds were designed through the online disulfide bond design website (http: / / cptweb.cpt.wayne.edu / DbD2 / index.php), namely E61C / N31C, E61C / T69C, I108C / A162C, G166C / G195C and G230C / V233C.
[0031] The construction of the disulfide bond mutant is roughly as follows (taking the mutant E61C / N31C as an example, and so on): using the constructed pPICZαA-ttl-1 as a template, first use the upstream and downstream primers E61C-fw and E61C-rev to carry out PCR amplification, agarose electrophoresis to de...
Embodiment 3
[0034] Example 3, Screening of Disulfide Bond Mutant Recombinant Yeast Engineering Strains
[0035] Pick the yeast recombinant transformants in Example 2 one by one with a toothpick to a 24-well plate containing 2 mL of BMGY medium in each well, culture at 30°C and 220 rpm for about 36 hours, and then add 0.75% (v / v) methanol to induce nourish. After culturing at 30° C. and 220 rpm for 24 hours, the supernatant was collected by centrifugation for enzyme activity determination. The lipase activity was determined by acid-base titration, and the instrument used was a pH-stat acid-base titrator (Swiss Metrohm China Co., Ltd.). The specific steps of enzyme activity determination are as follows: first, 5% (v / v) olive oil is added to 100mL water, and 2% (w / v) gum arabic is added as a stabilizer at the same time, and the olive oil substrate is obtained after high-speed homogenization for 5 minutes ; Take 15mL of olive oil substrate and add it to the reaction cup. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com