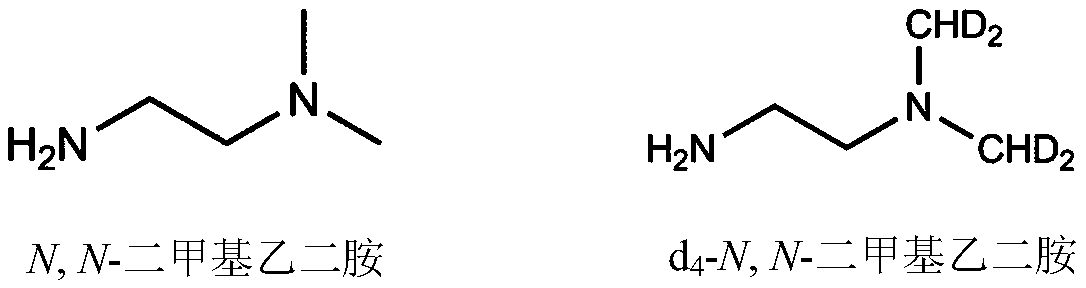Method for detecting carboxylic acid metabolites in plasma by using gas chromatography-mass spectrometry based on stable isotope labeling
A stable isotope, combined technology, applied in the field of detection of carboxylic acid metabolites in plasma based on stable isotope-labeled mass spectrometry, can solve the problems of low ionization efficiency and insufficient sensitivity, and achieve the goal of overcoming high prices and improving ionization. Low conversion efficiency and correction of matrix effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A method for detecting carboxylic acid metabolites in blood plasma based on stable isotope labeling mass spectrometry, the method is as follows:
[0056] (1) Preparation of plasma sample solution: 300 μL of ethyl acetate and 10 μL of 0.5% formic acid were added to 100 μL of plasma. After vortex mixing, centrifuge at 12000 rpm at 4°C for 5 min, extract, and collect the ethyl acetate layer. The extraction step was repeated three times, and the ethyl acetate layers obtained three times were combined, dried under nitrogen, and redissolved in acetonitrile as a sample solution for later use.
[0057] (2) Isotope labeling of the sample solution: take 200 μL of the sample solution in step (1), add 10 μL of 2 μmol / mL triethylamine and 10 μL of 120 μmol / mL 2-chloro-1-methylpyridinium iodide and mix well, Add 20 μL of 60 μmol / mL N,N-dimethylethylenediamine and shake at 50°C for 2 hours. After the reaction was completed, the reaction system was frozen at -20°C for 10 minutes to t...
Embodiment 2
[0094] 1. Screening of extraction solvent
[0095] The plasma used under this item is from the same source as the plasma used in Example 1 unless otherwise specified.
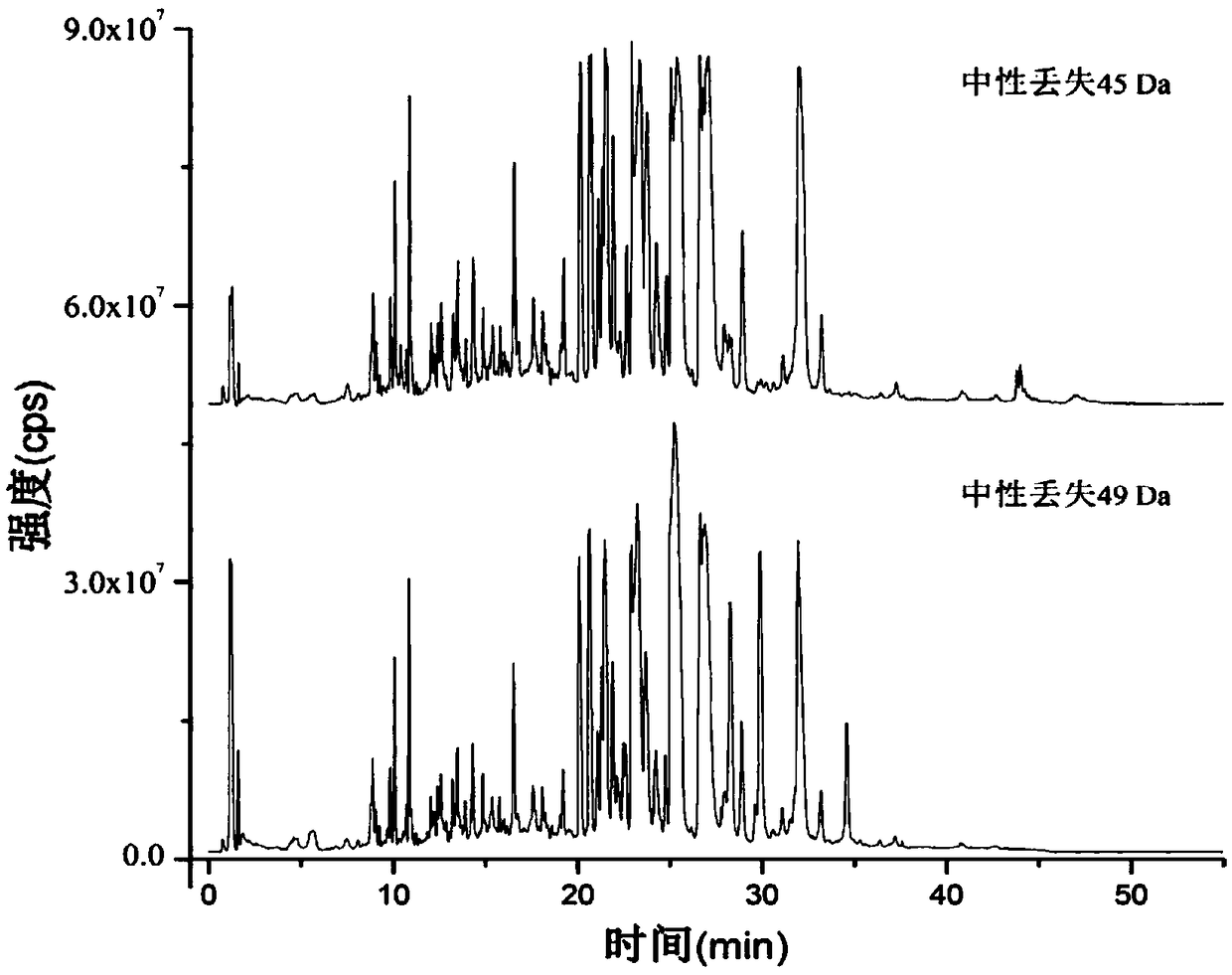
[0096] According to the method of Example 1, ethyl acetate (i.e. Example 1), acetonitrile, toluene, chloroform and n-hexane were used as extraction agents to analyze potential carboxylic acid metabolites in plasma respectively, because it is difficult to produce stratification when using acetonitrile to extract , when we used acetonitrile to test, we added an excess of sodium chloride to promote solvent separation.
[0097] The experimental results show that ethyl acetate, acetonitrile, toluene, chloroform and n-hexane can all extract carboxylic acid metabolites from plasma, but there are differences in the number of carboxylic acid metabolites that can be extracted. The quantities of carboxylic acid metabolites extracted respectively are shown in Table 3:
[0098] Table 3. The number of carboxylic acid metabol...
Embodiment 3
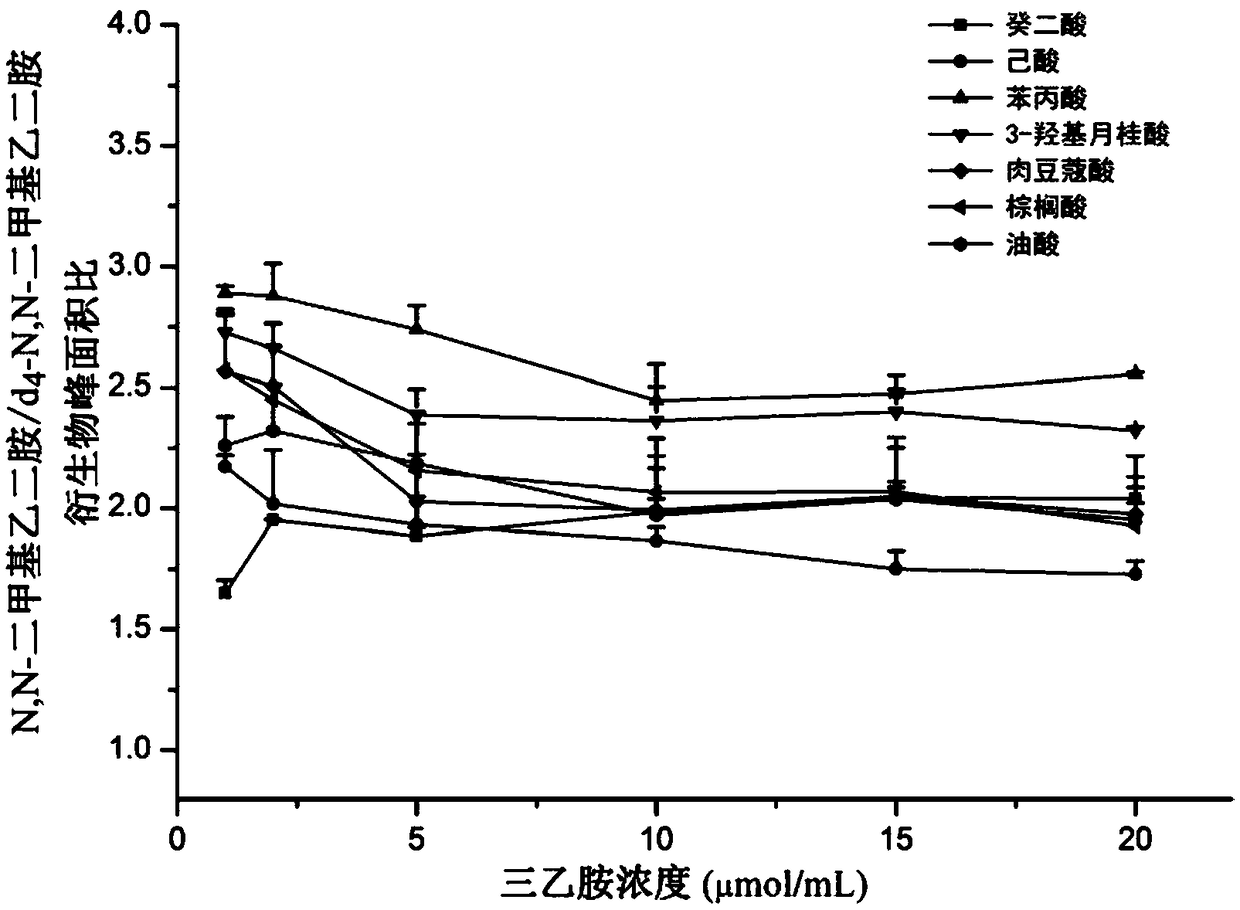
[0134] We use seven different types of carboxylic acid metabolites (sebacic acid, 3-hydroxylauric acid, phenylpropionic acid, caproic acid, myristic acid, palmitic acid and oleic acid) as model compounds and add them to the same mixture as in Example 1. In the plasma source, and verified according to the method of Example 1; As a result, in addition to the 269 carboxylic acid metabolites screened in Example 1, the carboxylic acid metabolites added this time were also analyzed as potential compounds come out.
[0135] In addition, we validated the experimental conditions of the present invention using the above known seven metabolites of carboxylic acids (sebacic acid, 3-hydroxylauric acid, phenylpropanoic acid, caproic acid, myristic acid, palmitic acid and oleic acid) , the specific process is as follows:
[0136] (1) Regarding catalyst concentration
[0137] According to the method in Example 1, we selected 1 μmol / mL, 2 μmol / mL, 5 μmol / mL, 10 μmol / mL, 15 μmol / mL and 20 μmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com