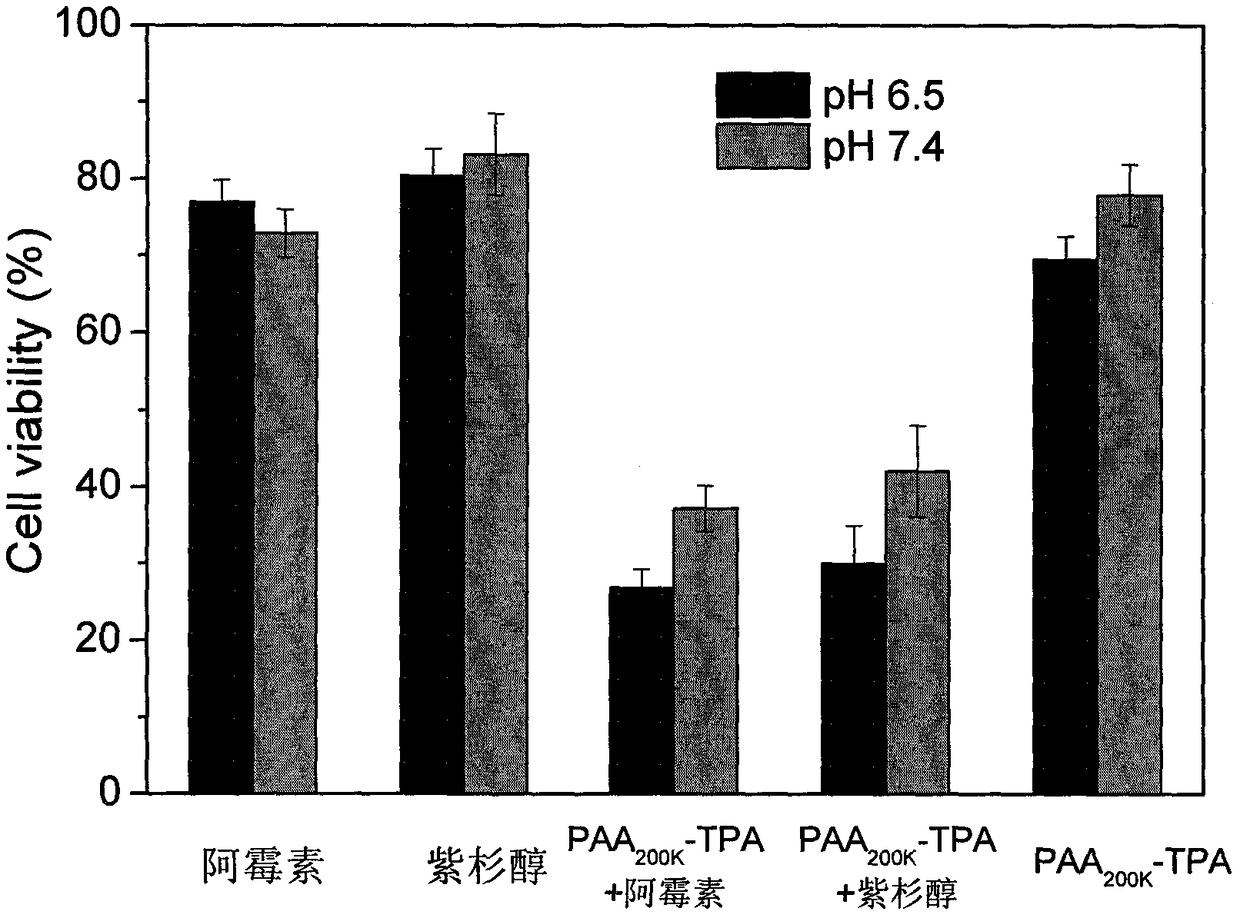
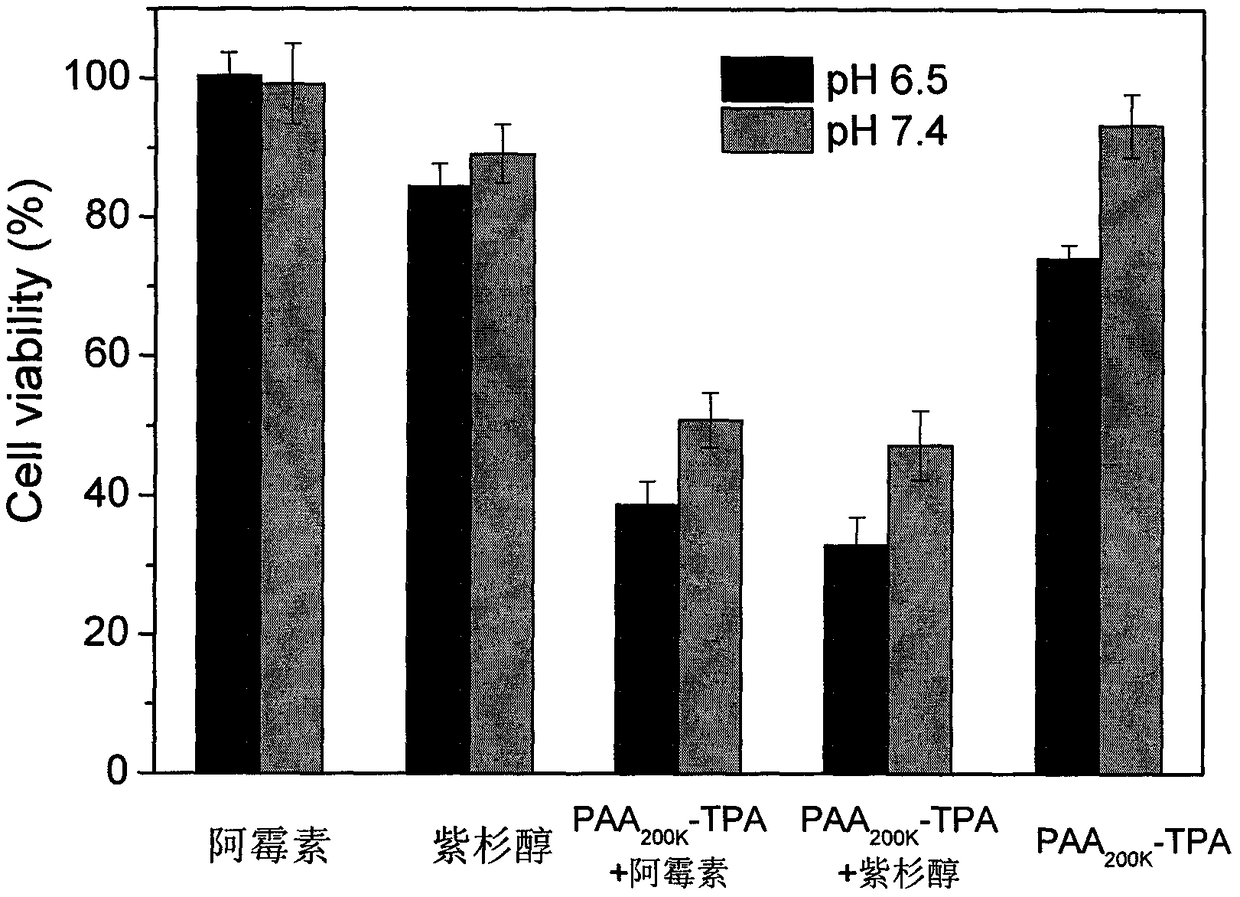
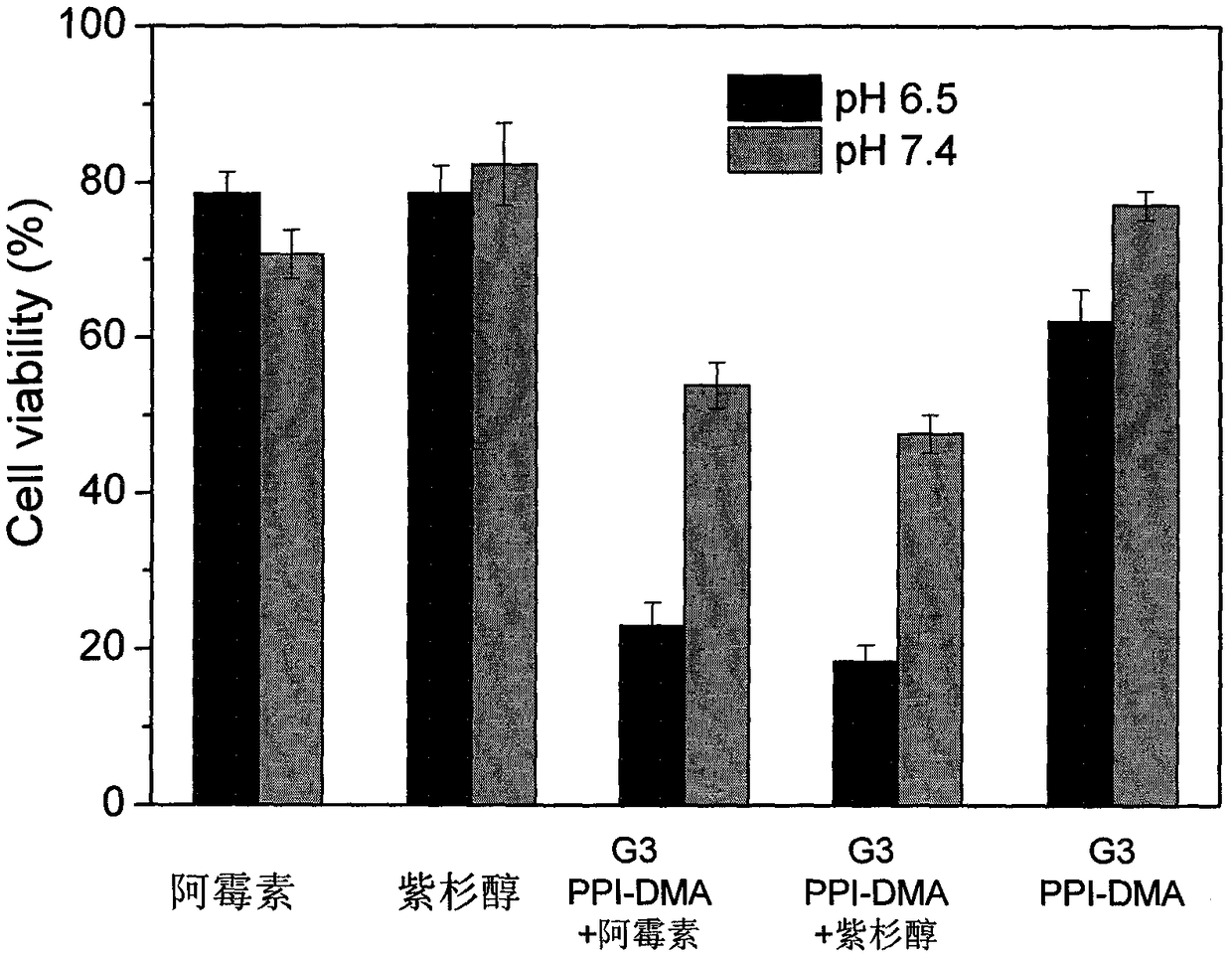
1,2-dicarboxylic acid mono-amide taken as chemotherapy synergist
A dicarboxylic acid monoamide and polymer technology, which is applied in the direction of drug combination, drug delivery, medical preparations of non-active ingredients, etc., can solve the problem of insufficient dosage, little application value, and influence on the particle distribution of macromolecular drug carriers and penetration issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 24 mg of 1,1,1-trimethylolpropane was weighed and added to 0.02 mL of 25% methanol solution of potassium methoxide, and excess methanol was distilled off under reduced pressure. Under the protection of nitrogen, slowly add 10ml of glycidol to the trimethylolpropane, the addition is completed in 10-12 hours, and the reaction is carried out at 95 degrees Celsius. After the reaction, the product was dissolved in methanol, potassium ions were removed by cation exchange resin, and then precipitated twice in ether to obtain hyperbranched polyglycerol, which was detected by gel permeation chromatography with a weight average molecular weight of 49,200. Dissolve 1 g of hyperbranched polyglycerol in 15 mL of tetrahydrofuran, add 4.3 g of p-toluenesulfonyl chloride and 4.5 mL of triethylamine, react at room temperature for 12 hours, remove the triethylamine salt by filtration, and then precipitate in ether to obtain p- Tosyl-modified hyperbranched polyglycerols. Dissolve it in 1...
Embodiment 2
[0029] The dendritic polyamide-amine (G8 PAMAM) of 0.6g 8 generations was weighed and dissolved in 10mL water, stirred and dispersed evenly in an ice-water bath, then adding 0.4g 2,3-dimethylmaleic anhydride ( DMA), the pH of the system dropped significantly after the addition of DMA, and sodium hydroxide solution was added to keep the pH of the reaction solution between 8-9. After the addition of DMA, the reaction was continued at room temperature for 24 hours. After the reaction, the reaction solution was added to the dialysis bag, and the pH was 10 in the sodium hydroxide solution for 72 hours, and freeze-dried to obtain 8 generations of dendritic polyamide-amine-2,3-dimethylmaleic anhydride Modifier (G8 PAMAM-DMA).
Embodiment 3
[0031] Weigh 0.6g of linear polyethyleneimine (LPEI) with a molecular weight of 10,000 10K ) was dissolved in 15mL water, stirred and dispersed evenly in an ice-water bath, and then 1.8g cyclohexene-1,2-dicarboxylic anhydride (TPA) was added in several times thereafter, the pH of the system dropped significantly after TPA was added, and sodium hydroxide was added The solution kept the pH of the reaction solution between 8-9, and continued the reaction at room temperature for 24 hours after adding TPA. After the reaction, the reaction solution was added to a dialysis bag, dialyzed in a sodium hydroxide solution with a pH of 10 for 72 hours, and freeze-dried to obtain a linear polyethylenimine-cyclohexene-1,2-dicarboxylate with a molecular weight of 10,000. acid modifier (LPEI 10K -TPA).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com