Organic photoelectric device containing oxathiocyclic triarylamine compounds and application thereof
A technology of optoelectronic devices and triarylamines, which is applied in the field of organic optoelectronic devices of oxygen-sulfur-containing heterocyclic triarylamine compounds, and can solve the problems of large influence on device yield, complex structure of OLED devices, and complex matching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
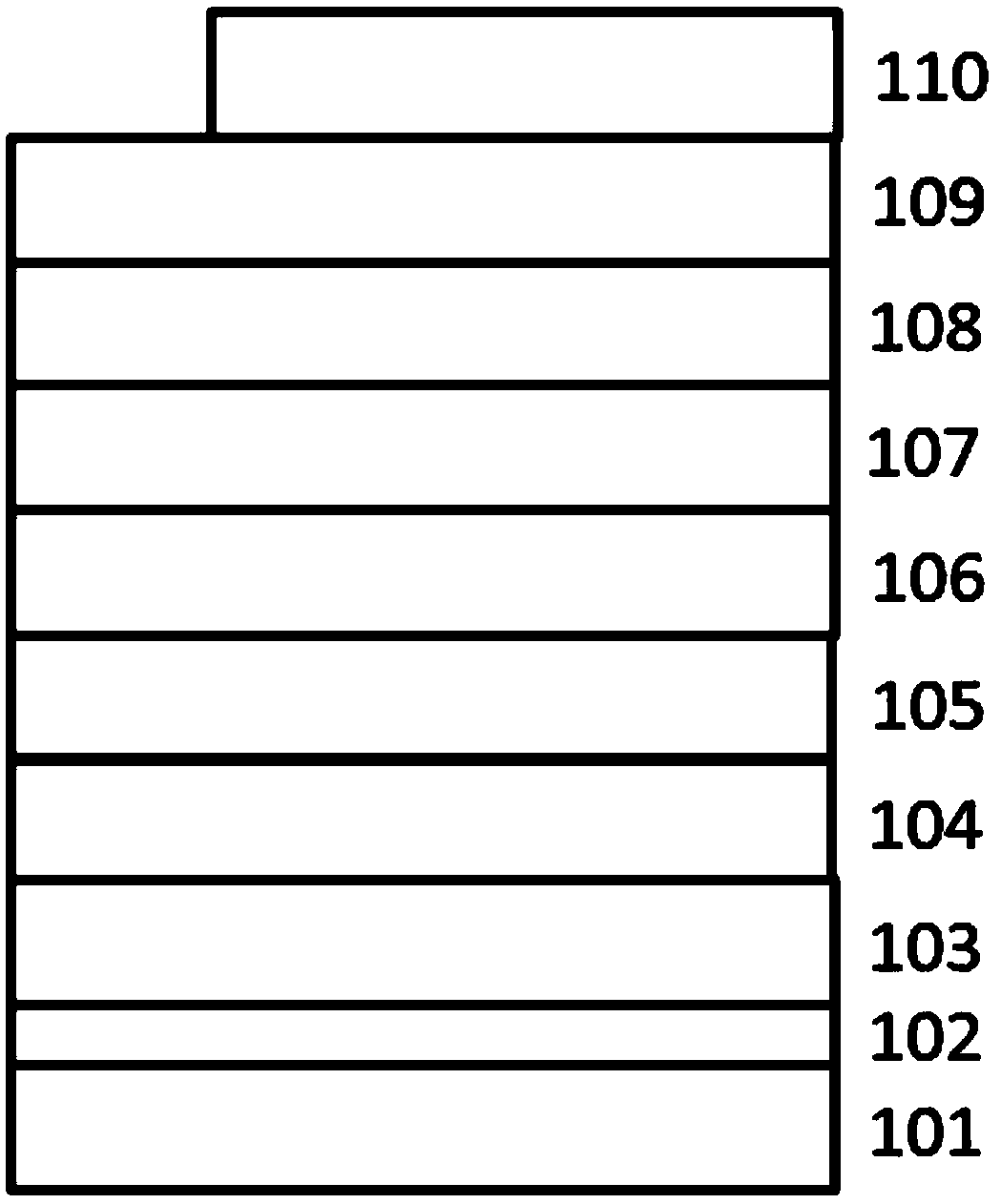
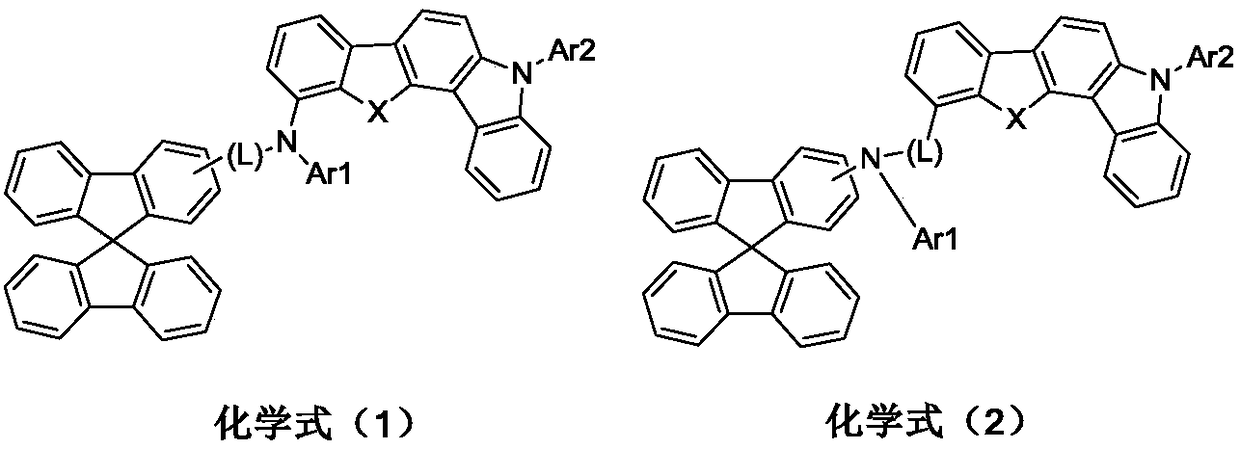
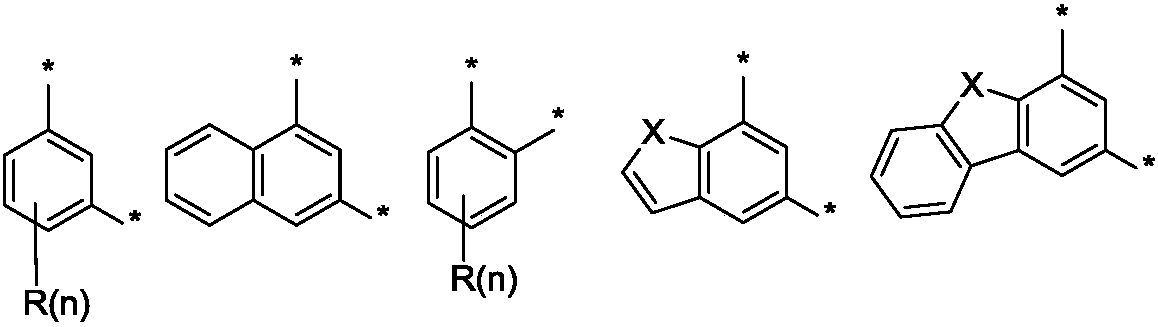
Image
Examples
Embodiment 1
[0084] Synthesis of intermediate oxygen-containing heterocycle-boronic acid intermediate O:
[0085]
[0086] Intermediate O-1 Synthesis
[0087]
[0088] 1) Dissolve dibenzofuran-4-boronic acid (S1) (2g) and 2-nitrobromobenzene (2g) in 60ml tetrahydrofuran completely in a 250ml round bottom flask under nitrogen atmosphere, then add 2M sodium carbonate aqueous solution After adding tetrakis-(triphenylphosphine)palladium (0.2g), the mixture was heated and stirred for 8 hours. After cooling to room temperature, the aqueous layer was removed. Add 100 ml of dichloromethane, and wash twice with 30 ml of saturated brine. The dichloromethane layer was dried over anhydrous magnesium sulfate, and concentrated in vacuo. Then petroleum ether: ethyl acetate (20:1~2:1) was used as eluent to purify and separate on a silica gel column to obtain S2 (yield 84%);
[0089]
[0090] 2) S2 (6g) and triethyl phosphite (16g) were completely dissolved in o-dichlorobenzene (120ml) in a 25...
Embodiment 2
[0103] Embodiment 2: the preparation of compound 3-1
[0104]
[0105] Dissolve spirobifluorene-2-boronic acid (6.8g) and MO-1 (10g) in 120ml toluene / tetrahydrofuran (1:1) completely in a 250ml round bottom flask under nitrogen atmosphere, then add 80ml of 2M sodium carbonate aqueous solution , and then tetrakis-(triphenylphosphine)palladium (0.2 g) was added, and the mixture was heated and stirred for 8 hours. After cooling to room temperature, the aqueous layer was removed. Add 200 ml of dichloromethane, and wash twice with 30 ml of saturated brine. The dichloromethane layer was dried over anhydrous magnesium sulfate, and concentrated in vacuo. Then use petroleum ether: dichloro (20:1 ~ 1:1) as eluent to purify and separate on a silica gel column to obtain compound 12.3 g (yield 75.8%). Measured MS (ESI): 815.2
Embodiment 3
[0106] Embodiment 3: the preparation of compound 3-11
[0107]
[0108] As shown in Example 1, after replacing MO-1 with MO-2, compound 3-11 was obtained with an isolated yield of 70.2%, and the measured MS (ESI): 891.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com