Method for preparing dolasetron mesylate key intermediate based on graphene activation
A technology of dolasetron mesylate and graphene, which is applied in organic chemistry and other fields, can solve problems such as a large impact on yield, achieve good antibacterial properties, high specific surface area, and improve reaction efficiency and activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
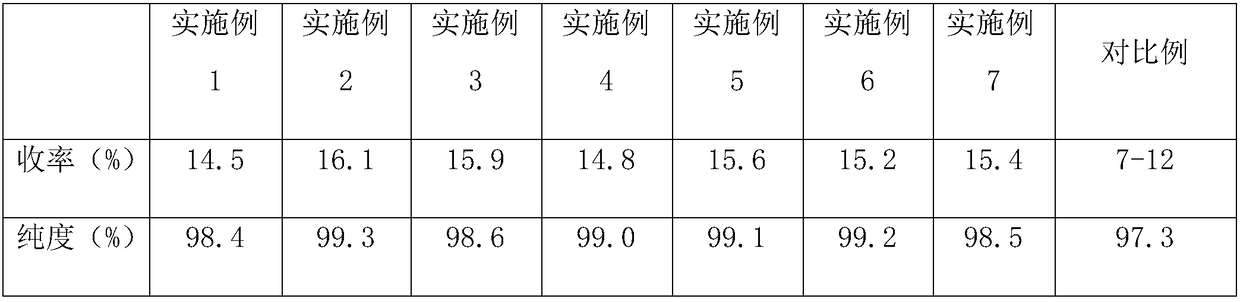
Examples
Embodiment 1
[0023] (1) According to the mass ratio of methyl 3-cyclopentene-1-carboxylate and N-methylmorpholine-N-oxide, potassium osmate, phenylboronic acid and sodium periodate is 1:0.1:0.05:0.05 : 0.3,3-cyclopentene-1-carboxylic acid methyl ester in the aqueous solution of N-methylmorpholine-N-oxide, after mixing evenly, first add potassium osmate and phenylboronic acid, and oxidize at 0°C for 15 minutes Afterwards, add sodium periodate again, and continue to heat up to 10°C at a rate of 1°C / min for 30 minutes of oxidation reaction to obtain CHOCH 2 -C-(COOCH 3 )-CH 2 CHO dialdehyde intermediate.
[0024] (2) Prepare 3-methoxycarboxy-7-oxo-9-azabicyclo-nonane-9-acetic acid methyl ester by reacting dialdehyde intermediate product with 1,3-acetone dicarboxylic acid by Robinson-Schoepf .
[0025] (3) According to the mass ratio of 3-methoxycarboxy-7-oxo-9-azabicyclo-nonane-9-acetic acid methyl ester and graphene oxide is 1:0.01, 3-methoxycarboxy-7 - In the tetrahydrofuran solution o...
Embodiment 2
[0027] (1) According to the mass ratio of methyl 3-cyclopentene-1-carboxylate to N-methylmorpholine-N-oxide, potassium osmate, phenylboronic acid and sodium periodate is 1:0.15:0.1:0.1 : 0.5, 3-cyclopentene-1-methyl carboxylate in the aqueous solution of N-methylmorpholine-N-oxide, after mixing evenly, first add potassium osmate and phenylboronic acid, and oxidize at 15°C for 60 minutes After that, sodium periodate was added, and the temperature was raised to 25°C for 45min at a rate of 3°C / min to obtain CHOCH 2 -C-(COOCH 3 )-CH 2 CHO dialdehyde intermediate.
[0028] (2) Prepare 3-methoxycarboxy-7-oxo-9-azabicyclo-nonane-9-acetic acid methyl ester by reacting dialdehyde intermediate product with 1,3-acetone dicarboxylic acid by Robinson-Schoepf .
[0029] (3) According to the mass ratio of 3-methoxycarboxy-7-oxo-9-azabicyclo-nonane-9-acetic acid methyl ester and graphene oxide is 1:0.1, 3-methoxycarboxy-7 - In the tetrahydrofuran solution of oxo-9-azabicyclo-nonane-9-aceti...
Embodiment 3
[0031] (1) According to the mass ratio of methyl 3-cyclopentene-1-carboxylate and N-methylmorpholine-N-oxide, potassium osmate, phenylboronic acid and sodium periodate is 1:0.12:0.06:0.08 : 0.4,3-cyclopentene-1-methyl carboxylate in the aqueous solution of N-methylmorpholine-N-oxide, after mixing evenly, first add potassium osmate and phenylboronic acid, and oxidize at 5°C for 30 minutes Afterwards, add sodium periodate again, continue to heat up to 15 ℃ oxidation reaction 35min at the rate of 2 ℃ / min, obtain CHOCH 2 -C-(COOCH 3 )-CH 2 CHO dialdehyde intermediate.
[0032] (2) Prepare 3-methoxycarboxy-7-oxo-9-azabicyclo-nonane-9-acetic acid methyl ester by reacting dialdehyde intermediate product with 1,3-acetone dicarboxylic acid by Robinson-Schoepf .
[0033] (3) According to the mass ratio of 3-methoxycarboxy-7-oxo-9-azabicyclo-nonane-9-acetic acid methyl ester and graphene oxide is 1:0.05, 3-methoxycarboxy-7 - In the tetrahydrofuran solution of oxo-9-azabicyclo-nonane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com