Preparation and application of a nanobiological dual-mimetic enzyme sensor
A technology that simulates enzymes and immunosensors. It is applied in biochemical equipment and methods, microbial measurement/inspection, instruments, etc. It can solve the problems of low catalytic efficiency and achieve long storage time, high detection sensitivity and good signal effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 immunosensor
[0026] (1) Synthesis of SiO 2 @Ni silicate core-shell structure nanomaterials
[0027] Add 0.8mL of 28% ammonia water to the mixture containing 36mL of ethanol and 5mL of distilled water, stir at room temperature for 10min, add 1mL of tetraethyl orthosilicate (TEOS), stir for 3h, and the product SiO 2 The nanospheres were centrifuged, and after washing, the SiO 2 Nanospheres were added to 6mL of absolute ethanol to disperse for later use.
[0028] Add 0.5g of cetyltrimethylammonium bromide (CTAB) to 75mL of distilled water, after it is completely dissolved, add 0.8mL of ammonia water, and then dropwise add 3mL of SiO prepared above. 2 Nanosphere ethanol solution, after ultrasonic dispersion, add 20mL n-hexane, stir at 30°C for 12min, then adjust the speed to 170rpm, slowly add 2.5mL TEOS dropwise, then continue to stir at 30°C for 9h, centrifuge the product after the reaction is complete Separation, after washing with a...
Embodiment 2
[0044] Example 2 Signal Amplification of Immunosensor
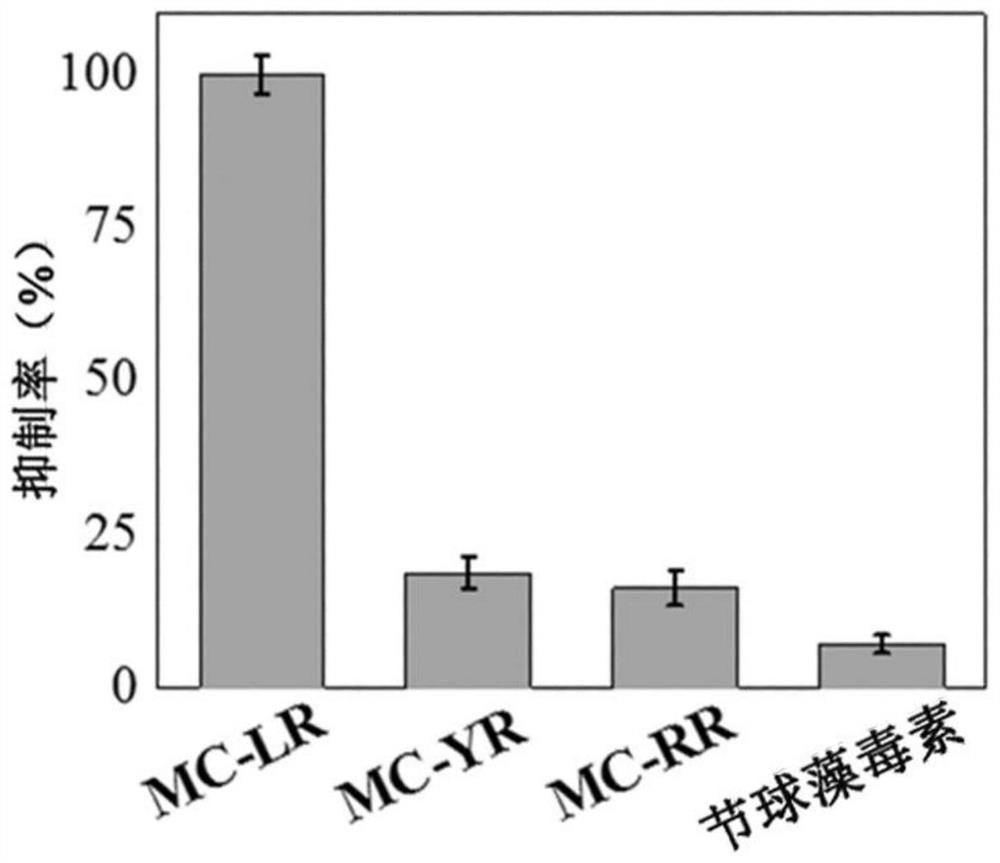
[0045] The invention constructs an immunosensor with multiple signal amplification effects by making full use of the surface effect of the nanometer material, the high catalytic ability and stability of the simulated enzyme, and the means of hybridization chain reaction (HCR) amplification. The immunosensor in the present invention uses aminated SiO 2 @Ni Silicate as the substrate, with Cu(OH) 2 SC-G-quadruplex nano-biological dual mimetic enzyme-labeled secondary antibody can greatly enhance the detection signal.
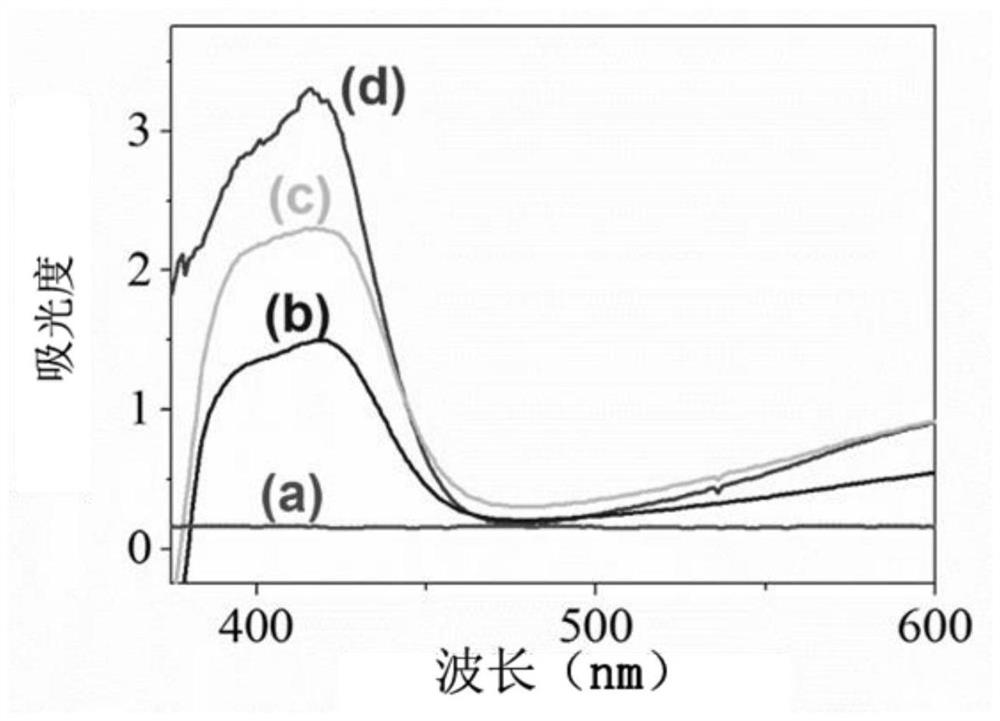
[0046] figure 1 It is the result of detection with different substrates and different secondary antibody markers. After immobilizing MC-LR antigens on the microplate, add MC-LR and Ab to be tested 1 Mixed solution, the color reaction of ABTS does not take place, and the ultraviolet-visible spectrophotometer also basically cannot detect any signal ( figure 1 a). When adding Cu(OH) 2 SC nanocage labeled A...
Embodiment 3
[0047] Example 3 Optimization of Immunosensor Detection Conditions
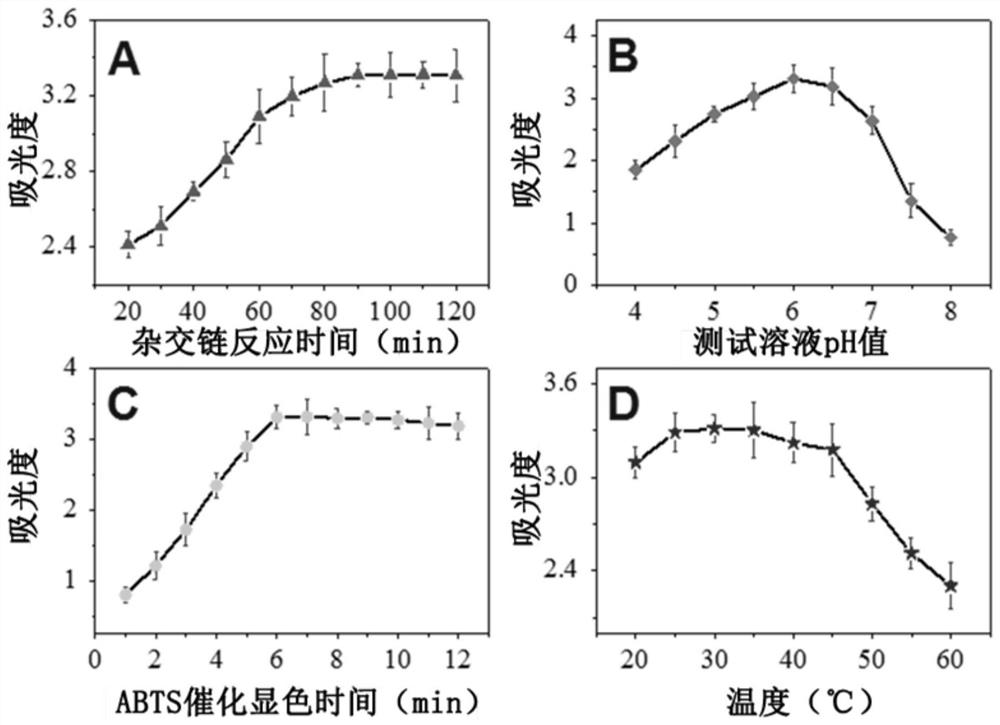
[0048] In order to improve the detection performance of the sensor, the effects of the hybridization chain reaction time, the pH value of the test solution, the catalytic color development time of ABTS and the catalytic temperature on the sensor signal were studied, and the best detection conditions were selected to optimize the performance of the sensor.
[0049] figure 2 A is the relationship curve between the sensor response signal (absorbance) and the hybridization chain reaction (HCR) time. As the HCR step time increases, the response of the sensor gradually increases and tends to be stable after 90 minutes, which indicates that the hybridization chain reaction is formed after 90 minutes. The length of the double-helix DNA does not increase, and the G-quadruplex on both sides of the double-helix DNA does not increase, so that the detection signal reaches a stable value, so the optimal HCR time for this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com