Amorphous carbon-loaded nano metal particle catalyst and preparation method and application thereof
A technology of nano-metal particles and amorphous carbon, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of hydrogen storage capacity loss and achieve distribution Uniform, conducive to capacity, hydrogen desorption kinetics performance and cycle performance improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
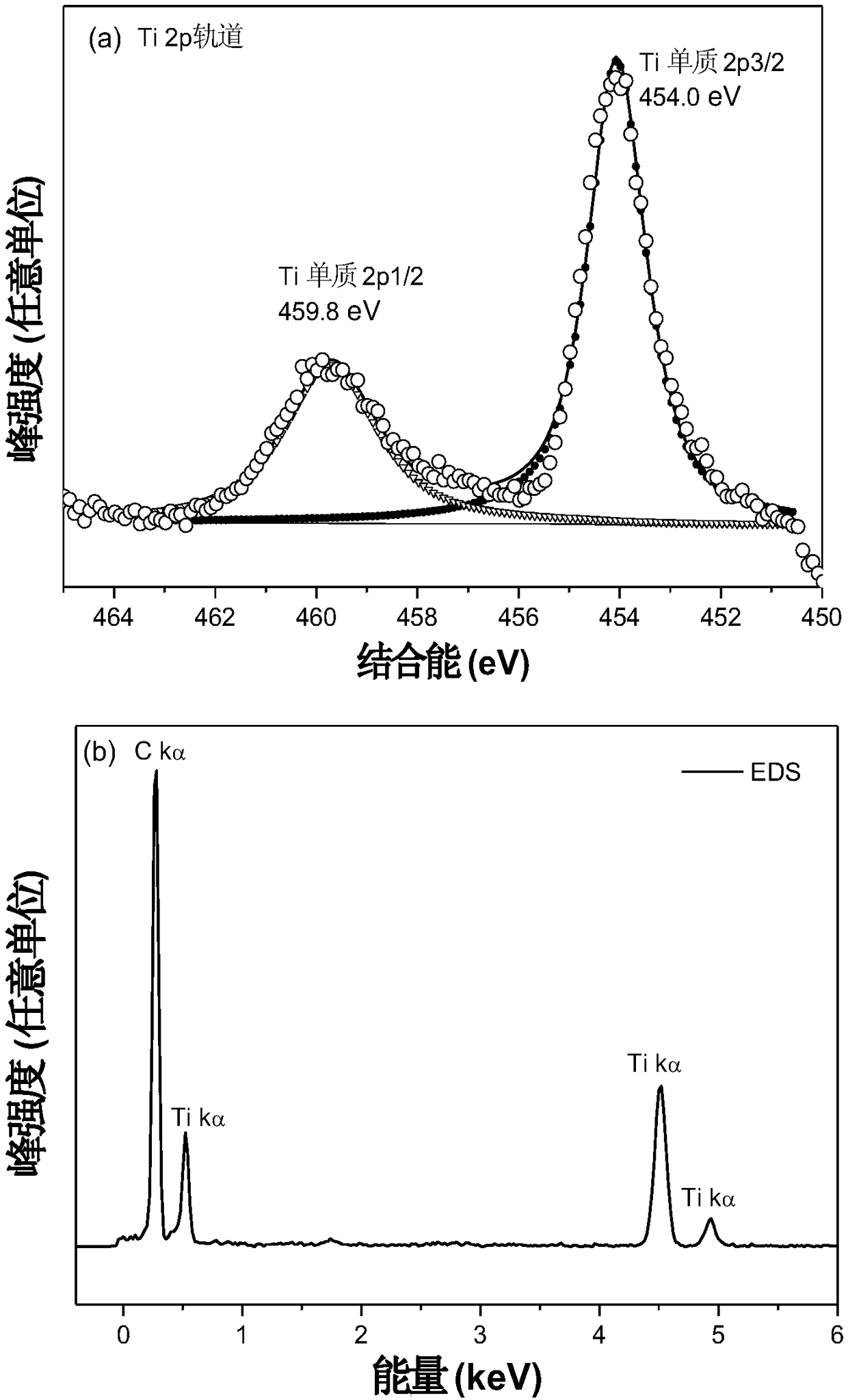
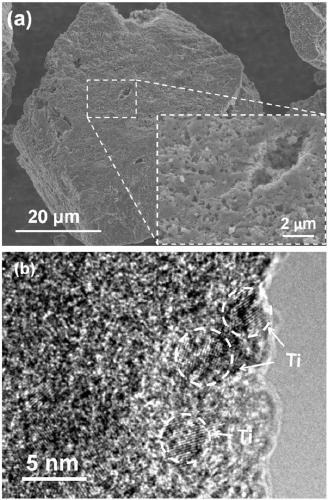
[0057] Preparation of nano-Ti@amorphous carbon composite catalyst:
[0058] (1) Titanocene dichloride (497.9 mg) and lithium hydride (31.8 mg) were put into a ball mill jar in an argon atmosphere glove box, mixed and ball milled at a speed of 300 rpm for 3 hours.
[0059] (2) Take out the uniformly mixed powder, put it into a quartz crucible, and raise the temperature to 550° C. for 2 hours under the protection of an inert atmosphere at a heating rate of 2° C. / min.
[0060] (3) Take out the heated solid powder in step (2) and put it into a 100ml flask and inject 50ml of ultra-dry pyridine, and magnetically stir for 2 hours to fully dissolve the by-product lithium chloride in the pyridine.
[0061] (4) The mixture in step (3) is filtered under reduced pressure in an inert atmosphere glove box, and the obtained solid powder is heated to 150°C for 5 hours under dynamic vacuum to remove residual pyridine, and it can be obtained after cooling Amorphous Carbon-Supported Nano-Metal ...
Embodiment 2
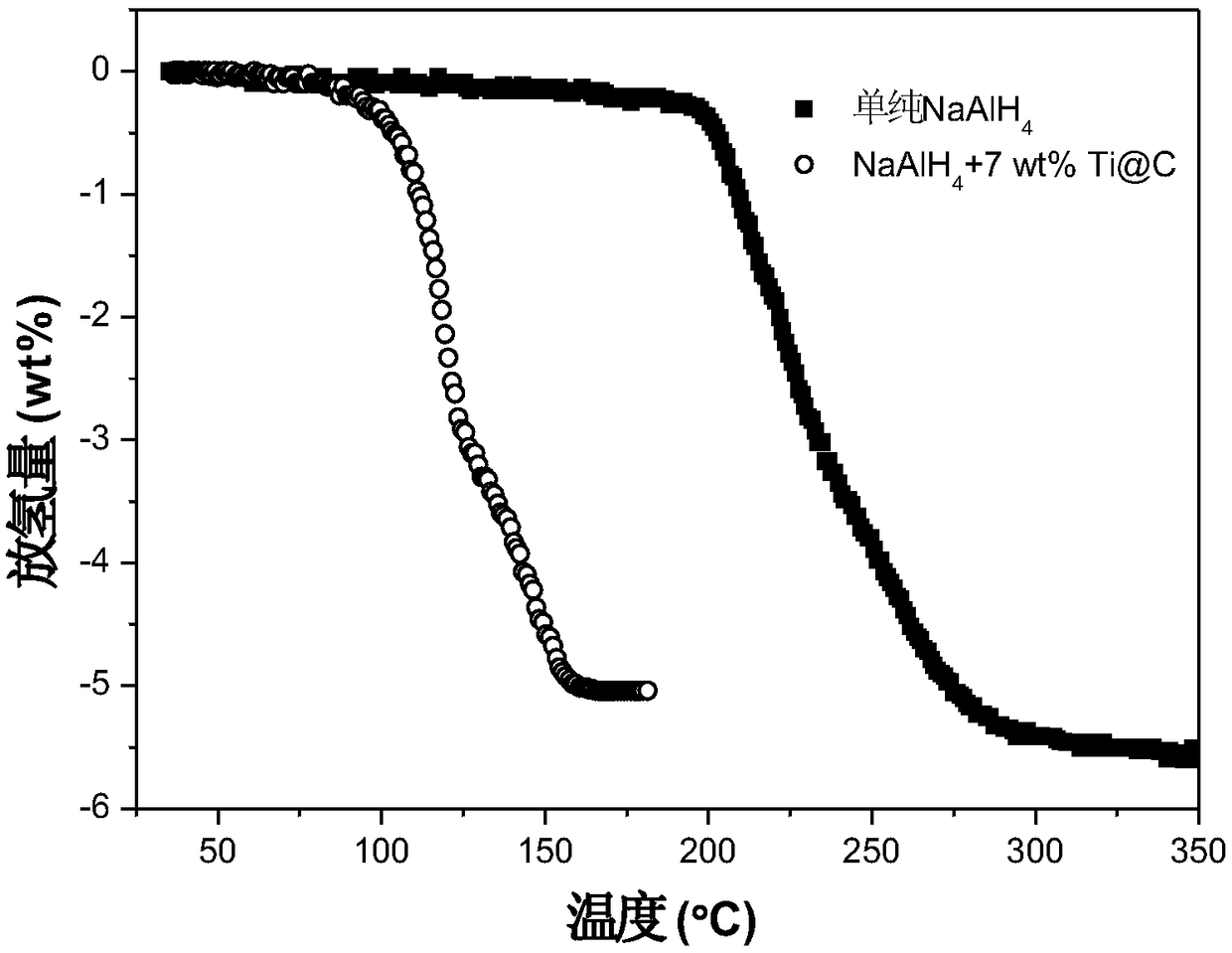
[0064] Nano-Ti@C Catalyzed NaAlH 4 Preparation of hydrogen storage materials: using the nano-Ti@C in Example 1 as a catalyst, NaAlH 4 The materials for hydrogen storage were mixed evenly in a certain proportion in an argon atmosphere glove box, and the mass fractions of Ti@C in the mixture were: 1wt%, 3wt%, 5wt%, 7wt% and 9wt%. Each mixture was placed in a stainless steel ball mill tank, and ball milled on a high-energy ball mill. The ball milling atmosphere was an argon atmosphere, the rotation speed was 500 rpm, the ball-to-material ratio was 120:1, and the ball milling time was 24 hours. Five copies of hydrogen storage materials, numbered respectively:
[0065] NaAlH 4 +1wt%Ti@C,
[0066] NaAlH 4 +3wt%Ti@C,
[0067] NaAlH 4 +5wt%Ti@C,
[0068] NaAlH 4 +7wt%Ti@C,
[0069] NaAlH 4 +9 wt% Ti@C.
[0070] The hydrogen desorption kinetics of five parts of hydrogen storage materials was tested by volumetric method. -3 Torr), heated to 250°C at a heating rate of 2°C / min...
Embodiment 3
[0081] The preparation process of the nano-Ti@C composite catalyst is the same as in Example 1.
[0082] Nano-Ti@C Catalyzed NaAlH 4 Preparation of hydrogen storage materials: using the nano-Ti@C in Example 1 as a catalyst, NaAlH 4 The matrix materials are mixed evenly in a certain proportion in an argon atmosphere glove box, and the mass fractions of Ti@C in the mixture are respectively: 7wt%. Each mixture was placed in a stainless steel ball mill tank, and ball milled on a high-energy ball mill. The ball milling atmosphere was an argon atmosphere, the rotating speed was 500 rpm, the ball-to-material ratio was 120:1, and the ball milling time was 24 hours. The hydrogen storage material is named: NaAlH 4 +7 wt% Ti@C.
[0083] The isothermal hydrogen desorption performance of the above hydrogen storage materials was tested by volumetric method. The dehydrogenation process is: under vacuum conditions (initial vacuum degree is 1×10 -3 Torr), heated to 140°C at a heating rate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com