Supported iron oxychloride Fenton's reagent and preparation method thereof
A technology of ferric oxychloride and Fenton's reagent, which is applied in chemical instruments and methods, oxidized water/sewage treatment, physical/chemical process catalysts, etc., can solve the problem of unstable metal oxide solid Fenton's reagent and Fenton's reagent Problems such as large amount of use and insufficient contact with the reaction solution achieve the effect of not easy to fall off and lose, the method is simple, and the effect of recycling is convenient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Clean the activated carbon to remove dust, put it into a 500ML beaker, add a sufficient amount of dilute hydrochloric acid solution (2.5mol / L) to boil, keep it for 2 hours, then add dilute hydrochloric acid solution of the same concentration to soak for 24 hours, remove the activated carbon and use it Wash with deionized water several times, and dry in an oven. Weigh 20 grams of ferric chloride hexahydrate and 5.5 grams of powdered activated carbon, heat them in an oven at 60°C for 20 minutes, place them in a constant temperature oscillator for 3 hours, and finally process them ultrasonically for 20 minutes. The mixture was placed in a closed container and heated at 200°C for 2 hours in a muffle furnace at a heating rate of 10°C / min. The obtained solid was ground into powder particles, washed several times with deionized water and acetone, and dried in an oven.
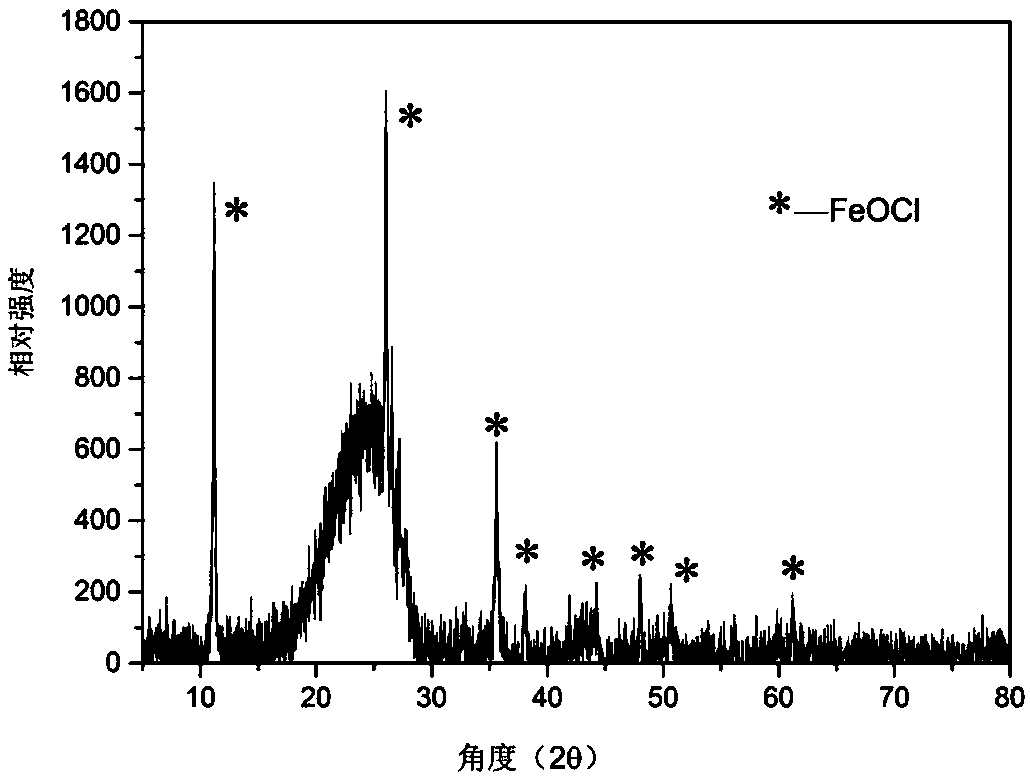
[0023] The Fenton's reagent prepared above was subjected to X-ray diffraction analysis, and it was known th...
Embodiment 2
[0026] The activated carbon pretreatment method is as described in Example 1. Weigh 10 grams of ferric chloride hexahydrate and 3.8 grams of pretreated coconut shell activated carbon particles, place them in an oven and heat them at 60 degrees for 20 minutes, and then place them in a constant temperature oscillator Shake for 3 hours, and finally sonicate for 20 minutes. The mixture was placed in a closed container, heated in a tube furnace at 250°C for 2 hours under a nitrogen atmosphere at a heating rate of 10°C / min, cooled to room temperature, and the solid was washed several times with deionized water and acetone, and placed in an oven drying.
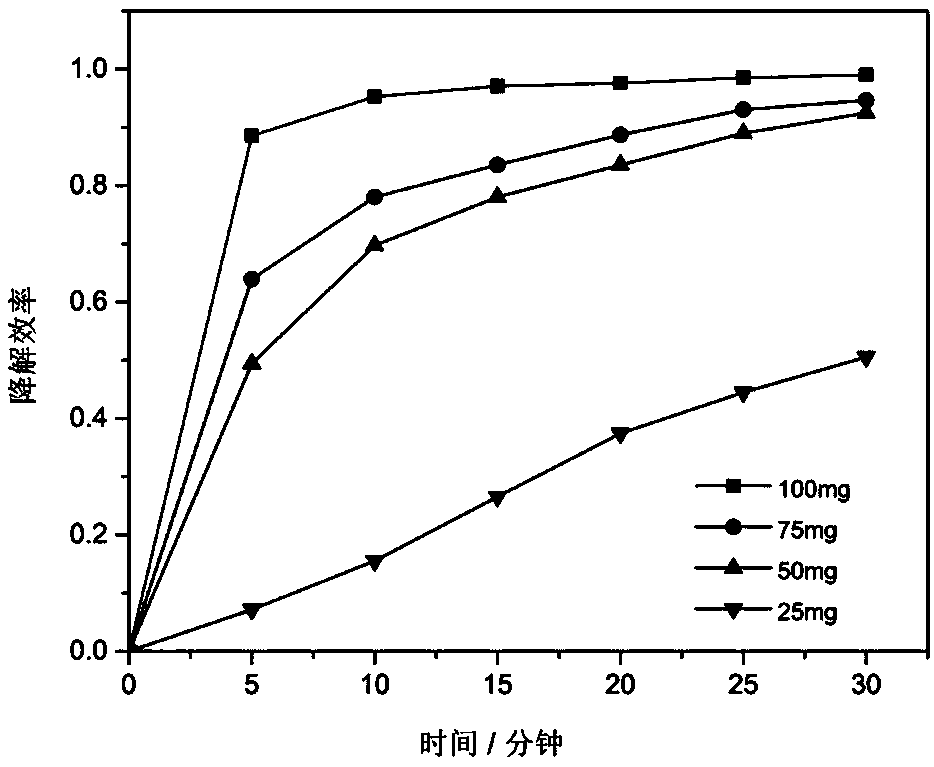
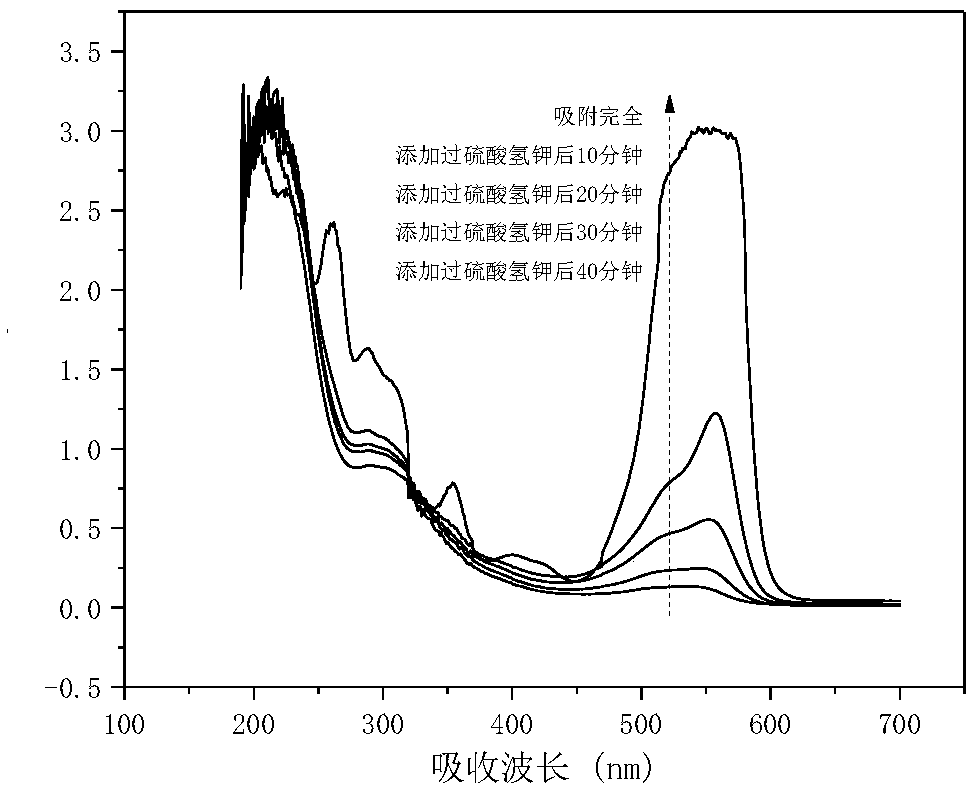
[0027] 100 mg of the product made in Example 2 is added to 100 milliliters of concentration in the rhodamine dye wastewater of 100 mg / L, the pH value is neutral, and the concentration of potassium persulfate added is 15 mmol / L, and the degradation rate within 40 minutes Up to 99%. The UV-Vis absorption spectrum changes with time d...
Embodiment 3
[0029] Add 100 mg of activated carbon-loaded oxyferric chloride Fenton reagent prepared in Example 1 into 100 milliliters of rhodamine dye wastewater with a concentration of 100 mg / L, adjust the pH value to between 9-10, and add potassium persulfate The concentration is still 15mmol / L, and the degradation rate can reach 90% within 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com