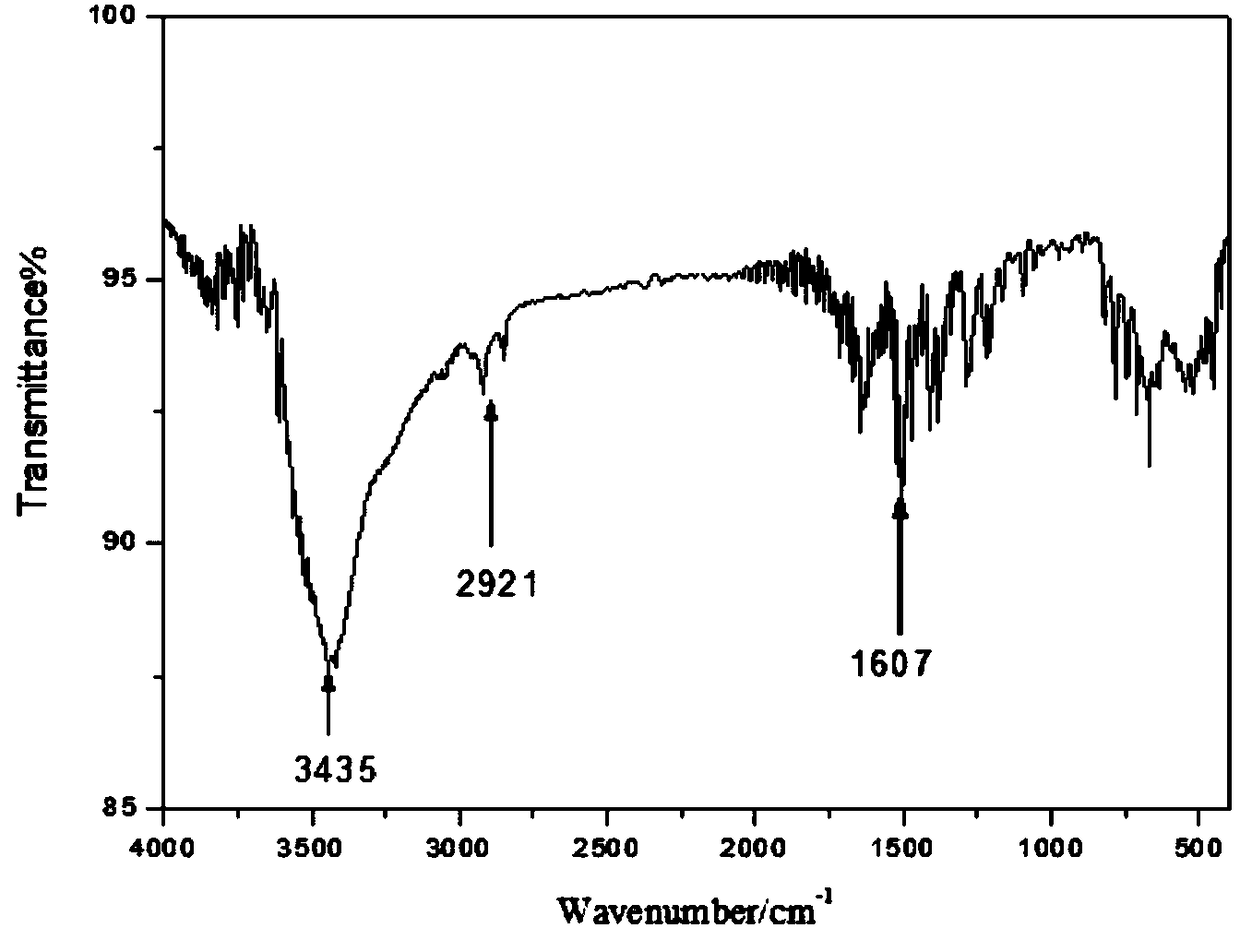
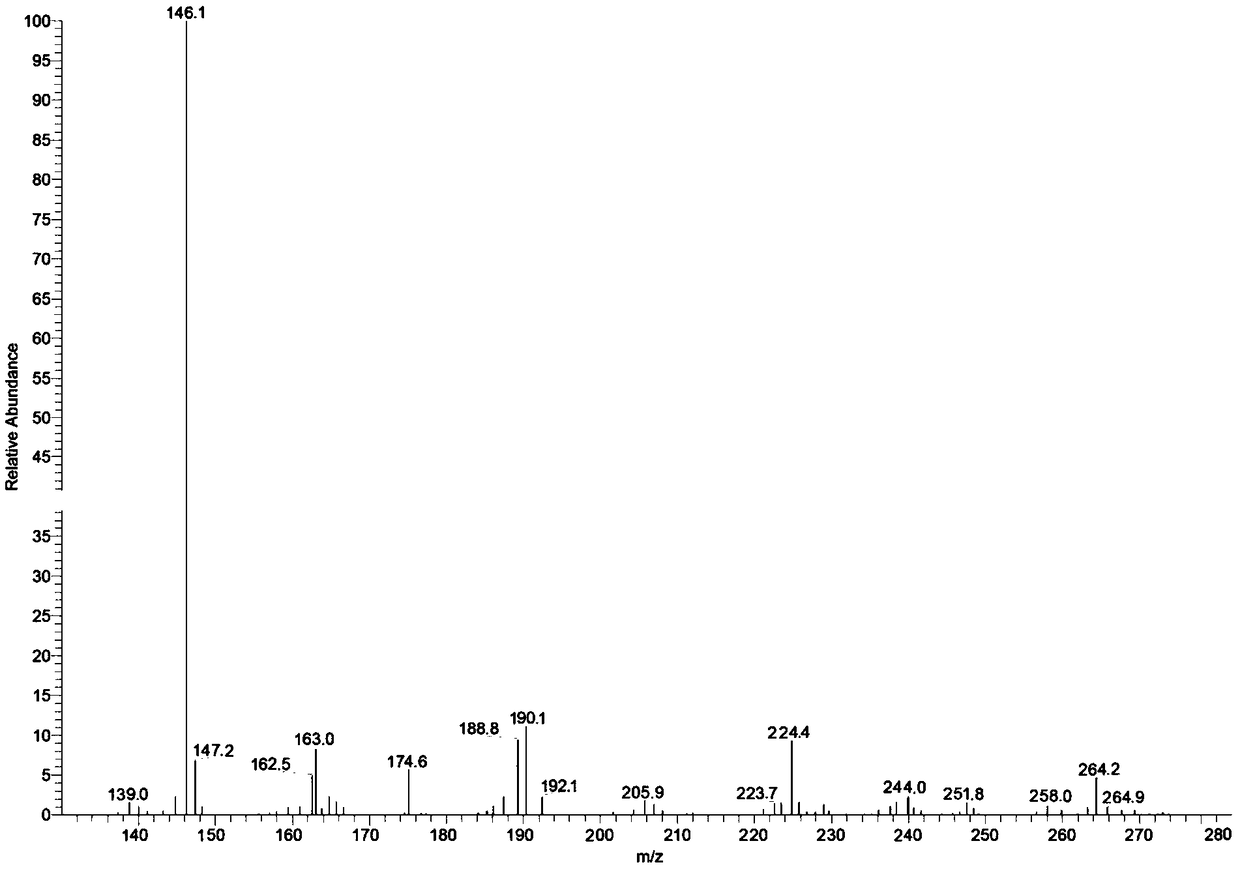
Method of synthesizing 8-hydroxyquinoline
A hydroxyquinoline and chelate technology, applied in the field of synthesizing 8-hydroxyquinoline, can solve the problems of being unsuitable for large-scale industrial production, difficult to control the reduction degree, unstable product yield and the like, and achieve easy control of the reaction process, The effect of stable yield and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Mix 45mL (1.5mol) hydrochloric acid, 3.3mL (0.6mol) glacial acetic acid, and 19.6g (0.18mol) o-aminophenol, stir and heat to 95-100°C, dissolve 1.0g hydroquinone in 12.0g (0.21mol) After acrolein, pour it into a constant pressure dropping funnel with a glass dropper connected to the lower end of the funnel, and slowly add it dropwise to below the liquid level of the reaction solution within 3 hours. It is measured that the filtrate only contains 18.4% of 8-hydroxyquinoline, which is directly used for the next photocatalytic dehydrogenation reaction without separation.
[0040] Add 3.0g (7.4mol×10 -3 mol)[PyCo(dmg) 2 Cl], 3.0g (4.3×10 -3 mol) Eosin Y, 50.0mL (0.96mol) of acetonitrile and 50mL (2.8mol) of deionized water, stirred, irradiated continuously with a 10W white LED lamp, and reacted at room temperature for 3 hours. After the reaction was completed, the acetonitrile was collected by vacuum distillation for reuse. Filtration, the filtrate is adjusted to pH=7, a...
example 2
[0042]Mix 45mL (1.5mol) hydrochloric acid, 3.3mL (0.6mol) glacial acetic acid, and 19.6g (0.18mol) o-aminophenol, stir and heat to 95-100°C, dissolve 1.0g hydroquinone in 12.0g (0.21mol) After acrolein, pour it into a constant pressure dropping funnel with a glass dropper connected to the lower end of the funnel, and slowly add it dropwise to below the liquid level of the reaction solution within 3 hours. Add 3.0g (7.4mol×10 -3 mol)[PyCo(dmg) 2 Cl], 3.0g (4.3×10 -3 mol) Eosin Y, 50.0mL (0.96mol) of acetonitrile and 50mL (2.8mol) of deionized water, stirred, continuously irradiated with a 10W white light LED lamp, reacted at room temperature for 3h, the reaction was completed, the reaction was completed, and the acetonitrile was collected by vacuum distillation Reuse, filter, add deionized water to the filtrate, steam distillation at normal pressure, collect the fraction at 97°C until the distillate has no white precipitate, distill the obtained filtrate to remove water under...
example 3
[0044] Mix 45mL (1.5mol) hydrochloric acid, 3.3mL (0.6mol) glacial acetic acid, and 19.6g (0.18mol) o-aminophenol, stir and heat to 95-100°C, dissolve 1.0g hydroquinone in 12.0g (0.21mol) After acrolein, pour it into a constant pressure dropping funnel with a glass dropper connected to the lower end of the funnel, and slowly add it dropwise to below the liquid level of the reaction solution within 3 hours. Add 0.5g (1.2mol×10 -3 mol)[PyCo(dmg) 2 Cl], 0.5g (7.2×10 -4 mol) Eosin Y, 50.0mL (0.96mol) of acetonitrile and 56g of the distilled raffinate of Example 1, stirred, continuously irradiated with a 10W white light LED lamp, and reacted at room temperature for 3h. , the filtrate is adjusted to pH=7, and the suction filtration filter cake under reduced pressure is a thick product, and the filtrate is distilled under reduced pressure to remove water. For the next photocatalytic reaction. The crude product was distilled under reduced pressure, the vacuum pump negative pressur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com